1. Department of Neurobiology Key Laboratory for Neurodegenerative Disease of the Ministry of Education, Capital Medical University, Beijing 100069, China;
2. Department of Cell Biology, Capital Medical University, Beijing 100069, China
N-acetylcysteine protects against apoptosis induced by overexpressed mGlu1a in agonist-dependent and-independent pathways
WANG Shu-ting1,2, XIA Ning1,2, YUAN Ji-fang1,2, ZHANG Hong1,2
Abstract
Objective To investigate the effects of N-acetylcysteine (NAC ) on constitutive (agonist-independent) and agonist-stimulated mGlu1a-mediated excitotoxicity associated with mGlu1a overexpression in HEK293 cells. Methods The signaling pathways, cell viability, the surface expression of mGlu1a and the intracellular oxidative stress were detected by western blot, MTT, trypan blue-exclusion assay,Elisa, Fluorescence-based Detection of Cellular ROS and HPLC methods. Results In mGlu1a-transfected cells and mGlu1a-transfected, DHPG-induced cells, NAC inhibited the excitotoxicity of mGlu1a by different mechanisms. Under two conditions, NAC prohibited the production of ROS and modulated the intracellular glutathione redox potential. Conclusion This study further suggested that the complex effects of mGlu1a activity under different conditions might play different role in the progress of many diseases, especially in terms of the effects of increased receptor expression.
NAC通过不同机制抑制激动剂依赖和非依赖的过表达mGlu1a介导的细胞凋亡
王舒婷1,2, 夏宁1,2, 袁记方1,2, 张红1,2
1. 首都医科大学神经生物学系, 教育部神经变性疾病重点实验室, 北京 100069;
2. 首都医科大学细胞生物学系, 北京 100069
2. 首都医科大学细胞生物学系, 北京 100069
摘要
目的 探测N-乙酰半胱氨酸(NAC)对于组成型和诱导型表达mGlu1a所介导的兴奋性毒性的影响。 方法 在过表达mGlu1a的HEK293细胞中,通过免疫印迹法,MTT法,胎盘蓝排斥法,酶联免疫吸附实验,二氯荧光素检测法及HPLC等方法,探测了NAC对mGlu1a下游信号分子活性,细胞活力和凋亡,受体表面表达以及细胞内氧化应激的影响。 结果 发现受体的组成型和诱导型活性通过不同机制,参与了NAC对于mGlu1a所介导的兴奋性毒性的抑制。在以上两种情况下,NAC均可以通过降低ROS调节细胞内氧化还原电势。 结论 在不同生理刺激条件下,mGlu1a的活性对于疾病的发生可能起着不同的作用,尤其是对mGlu1a高表达所产生的效应,为探究与mGluI相关疾病的发生提供了理论依据。
内容大纲
-
1 材料与方法
- 1.1 材料与试剂
- 1.2 细胞培养与转染
- 1.3 免疫印迹法(Western blot)
- 1.4 酶联免疫吸附实验定量检测受体表面表达
- 1.5 细胞活力检测
- 1.6 细胞内ROS生成检测
- 1.7 HPLC法检测血浆中GSH/GSSG氧化还原电势
- 1.8 统计学分析
mGluI的活性分为组成型(激动剂非依赖型 )和诱导型(激动剂依赖型)。根据激活的条件不同,mGluI可以引起相关的细胞毒性或细胞保护效应。如:mGluI可以通过氧糖剥夺加剧神经元的死亡[6],亦可通过抑制活性氧(ROS)的聚集和细胞内谷胱甘肽的丧失而减轻少突细胞的兴奋性毒性[7]。在HEK293细胞中瞬时转染mGlu1可引起激动剂非依赖的细胞死亡,共表达G蛋白耦联受体(GPCR)激酶2可起到保护作用[8]。mGluI可通过调节包括ERK通路在内的多种细胞内信号转导途径,参与mGluI所介导的神经元保护[9]或毒性作用[10]。 mGluI激动剂DHPG可引起皮层细胞中PARP活性的显著升高,这种效应可被mGlu1拮抗剂3-MADITA完全消除,并被mGlu5拮抗剂MPEP部分抑制[11]。此外,mGluI表面表达的调控也对神经元功能起到了关键的作用[12]。由于mGluI在不同生理条件下的作用不同,探讨对其作用的调控非常重要。
有研究表明,在C6细胞,MN9D细胞以及大鼠帕金森疾病模型中,N-乙酰半胱氨酸(NAC)(抗氧化剂谷胱甘肽GSH的前体)通过对mGluI活性的不同调节作用而起到神经元保护作用[13]。为了更好地理解mGluI在NAC发挥作用过程中的功能,本研究在293细胞中瞬时转染mGlu1a,利用激动剂依赖型和非依赖型受体活性效应,探测NAC对mGluI高表达与其活性关系的调节机制,进一步了解mGluI在不同生理条件下的激活机制。
1 材料与方法
1.1 材料与试剂 DMEM ( Dulbecco modfied eagle medium )培养基和胎牛血清购买于Hyclone 公司;DHPG购于Tocris 公司;NAC和丁硫氨酸亚砜胺(BSO)购买于Sigma公司,抗体PARP,β-actin,Bcl-2,Bax购于Cell Signaling公司;p-ERK及ERK购于Millipore公司,Flag M2购于Sigma公司,Flag-mGlu1a质粒由Randy Hall提供。
1.2 细胞培养与转染
HEK293细胞在含10%胎牛血清及1%氨苄西林和1%硫酸链霉素的DMEM(高糖)培养基中,37 ℃,5% CO2 培养箱中培养。为了实现mGlu1a的过表达,按照普利莱基因技术有限公司转染试剂Hifectin Ⅱ的操作手册将pcDNA3.1-flag-mGlu1a质粒转染至HEK293细胞。
1.3 免疫印迹法(Western blot)
取适量样品于浓度为8%~15%的十二烷基硫酸钠-聚丙烯酰胺凝胶(SDS-PAGE, polyacrylamide gel ectrophoresis )上进行电泳,电泳结束后将凝胶中的蛋白转移到PVDF膜上,通过Bio Radmini-ProteinⅡ电转系统(美国Bio RAD公司)进行湿转,之后用5%的脱脂奶粉室温封闭1 h,加入相应稀释过的一抗,4 ℃孵育过夜,TBST缓冲液洗膜,7 min × 4次,二抗经稀释(1 ∶ 3000)后,室温孵育1 h,TBST缓冲液洗膜,7 min × 4次,使用化学发光液进行蛋白水平检测。
1.4 酶联免疫吸附实验定量检测受体表面表达
该方法已经过相关报道证实[14],简单来说,首先将经过Flag-mGlu1a转染的HEK293细胞接种到96孔板上,使用HBSS缓冲液洗细胞使其平衡,将细胞用DHPG预处理5 min后加入NAC处理30 min,然后用含有4%多聚甲醛的PBS溶液将其固定,再用PBS冲洗细胞,之后由牛血清白蛋白(BSA)溶液封闭非特异性结合位点,加入小鼠来源的Flag一抗(CST),孵育完毕后加入抗鼠IgG-HRP二抗(CST),细胞洗净后加入HRP底物邻-苯二胺(OPD)1 mg/ml,同体积0.1 N的H2SO4用来中止反应,最后用酶标仪在492 nm处检测每个孔的吸光度。
1.5 细胞活力检测
HEK293细胞活力通过MTT以及胎盘蓝排斥方法检测,具体方法在本实验室之前的文章中有较为详细的描述。对于MTT比色法,先将HKE293细胞接种到96孔板中,经过合适的药物刺激处理后,弃去原培养基,每孔加入含有5 mg/ml MTT的无血清培养基,37 ℃,5%CO2培养箱中培养4 h,弃去含MTT的无血清培养基,加入100 μl二甲基亚砜(DMSO),振荡10 min,使紫色结晶溶解,酶标仪490 nm检测活力。对于胎盘蓝排斥法,需将经过药物处理的细胞于4%台盼蓝(Sigma)溶液中染色5 min,未染色的细胞数与细胞总数之比可作为细胞活力的指标。
1.6 细胞内ROS生成检测
收集不同作用条件下的各组细胞,吸除培养液,加入无血清培养液稀释DCFH-DA,使DCFH-DA终浓度为10 μmol/L,继续培养30 min后使用酶标仪(Thermo, USA)检测荧光强度,以488 nm激发波长和530 nm发射波长设置参数。
1.7 HPLC法检测血浆中GSH/GSSG氧化还原电势
该方法在之前的文章中已有介绍[13],细胞经PBS洗后给予含有0.2 mol/L硼酸和10 μmol/L γ-Glu-Glu的5%高氯酸溶液,处理后的细胞可收集并储存于-20 ℃。测定前将样品取出,并用硼乙酸和丹磺酰氯使其衍生化,衍生化的样品离心后取上清应用于Supelcosil LC-NH2硅胶柱,并通过梯度洗脱将其分离,最后检测其荧光强度。GSH/GSSG(Eh)的氧化还原电势由GSH与GSSG的摩尔浓度经能斯特方程计算而来,方程如下:
GSH/GSSG (Eh)=-264+30log( / 2)
1.8 统计学分析
所有数据被表示为X ±Sx 。采用单因素方差分析,P<0.05为差异有统计学意义。
2 结果
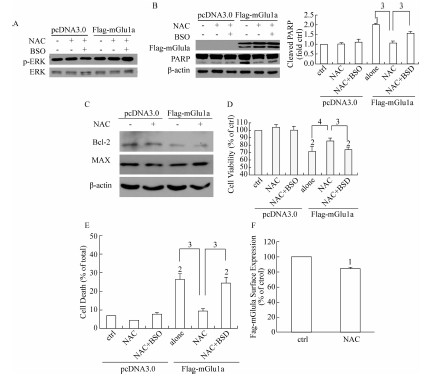
2.1 NAC对过表达mGlu1a所引起的细胞毒性的作用由于HEK293细胞转染效率较高,因而本文采用HEK293细胞作为研究过表达受体作用的体系。HEK293细胞中瞬时转染mGlu1a可引起激动剂非依赖型的细胞死亡[8],因此该研究希望探测NAC对组成型mGlu1a活性的影响。实验采用HEK293细胞,转染mGlu1a 48 h后给予5 mmol/L NAC 12 h,通过Western免疫印迹方法,检测在mGlu1a引起毒性作用过程中,其下游信号分子ERK的磷酸化是否参与。结果显示,与对照组相比,NAC不能引起p-ERK水平 的改变(图1A)。以Bcl-2/Bax比例和剪切的PARP作
图1 NAC在过表达mGlu1a的HEK293细胞中的作用
Figure 1 Effect of NAC on mGlu1a overexpressing HEK293 cells 注: A: HEK293 cells were transfected with pcDNA3.0-Flag-mGlu1aor empty plasmid as a control. At 48 h after transfection, cells were treated with 5 mmol/L NAC, with or without 100 μmol/L BSO pre-treatment. Western blotting was performed to detect phosphorylation of ERK; B: At 36 h after transfection, cells were treated with 5 mmol/L NAC for 12 h in the presence or absence of BSO. Representative Western blots show the cleavage of PARP (left panel). Densitometric analysis shows cleaved PARP for each condition, compared with control (left lane, right panel); C:At 48 h after transfection, cells were treated with 5 mmol/L NAC, with or without 100 μmol/L BSO pre-treatment. Western blotting was performed to detect levels of Bcl-2/Bax; D: At 48 h after transfection of mGlu1aor empty plasmid, HEK293 cell viability was measured by MTT assay. Cell viability is expressed as a percentage of the control culture (left column); E:NAC inhibited cell death caused by mGlu1ain HEK293 cells. At 36 h after transfection, cells were treated with 5 mmol/L NAC for 12 h in the presence or absence of BSO. Cell death was evaluated by trypan blue-exclusion assay, and expressed as a percentage of total cells; F: An ELISA-based assay was used to detect changes in surface expression of mGlu1ain HEK293 cells with or without NAC treatment. Surface expression is expressed as a percentage of the control culture (left column). (1)P<0.05 and (2)P<0.01 versus control; (3)P<0.05, (4)P<0.01.
为凋亡指标,检测NAC对mGlu1a所导致的细胞凋亡的影响。发现与对照组相比,NAC可以降低PARP的剪切,并且各组mGlu1a的转染效率基本一致(图1B)。为了进一步证实NAC的作用,实验利用γ-谷氨酰胺半胱氨酸合成酶抑制剂BSO,抑制GSH的合成,以此消除NAC的还原作用。经100 μmol/L BSO预处理30 min后,再给予NAC。结果显示,与未经BSO预处理组相比,BSO对ERK磷酸化没有明显影响(图1A),但可抑制PARP的剪切(图1B),提示在组成型mGlu1a引起细胞毒性作用的过程中,谷胱甘肽的抗氧化功能参与了NAC的细胞保护作用。在此过程中Bcl-2/Bax比例与对照组差异均无统计学意义 (图1C),进一步用MTT法检测细胞活力的改变,结果显示,在HEK293细胞中,过表达mGlu1a能够降低细胞的活力,与单独过表达组相比,NAC可以缓解细胞活力的下降,BSO预处理后可以反转NAC的这种作用(图1D)。通过胎盘蓝染色法测定细胞死亡,进一步证实了NAC能够部分抑制mGlu1a引起的细胞死亡(图1E)。由于内化作用,mGlu1/5的激活可以导致mGlu1/5在细胞表面的表达量下降[15],通过酶联免疫吸附测定实验,本文研究结果显示,NAC可使mGlu1a细胞表面表达量下降17%(图1F)。以上结果提示,NAC具有对抗组成型mGlu1a所诱导的细胞毒性作用的功能,ERK, Bcl-2,Bax独立于该过程,而NAC对于mGlu1a细胞表面表达量的影响可能参与了该过程。
2.2 NAC对DHPG激活过表达mGlu1a引起的细胞毒性的作用
与不依赖于激动剂的受体组成型活性不同,DHPG激活mGlu1a后可引起ERK磷酸化[16]。已经研究证实,在C6和MN9D细胞中,通过调节mGluI活性,NAC可以缓解由毒性试剂所引起的细胞的凋亡[13]。为了研究NAC对过表达mGlu1a激活所发挥的作用,在HEK293细胞中转染mGlu1a,加入100 μmol/L DHPG预处理5 min,再给予NAC处理12 h,通过Western免疫印迹法检测DHPG激活mGlu1a后,NAC对ERK磷酸化的影响。结果显示,DHPG可以促进细胞内ERK的磷酸化,而NAC可以缓解由DHPG所引起的ERK磷酸化。为了进一步确证NAC在DHPG对ERK活性调节过程中的作用,实验采用BSO预处理细胞后检测ERK磷酸化,发现BSO可以部分恢复由NAC所引起的效应,提示GSH的抗氧化作用参与了mGlu1a对于ERK磷酸化的调节过程。接下来,实验进一步探测了过表达mGlu1a 的HEK293细胞中,NAC对细胞凋亡的影响状况。结果显示,与对照组相比,DHPG可以降低Bcl-2/Bax的比例,再给予NAC后可部分缓解DHPG的作用,而NAC的这种保护作用在BSO预处理的细胞中有所减轻,显示了NAC具有对抗过表达mGlu1a激活所导致的细胞毒性作用的功能 (图2A)。此外,NAC对于DHPG所引起的mGlu1a细胞表面表达水平的降低没有明显影响(图2B)。综上所述,在过表达mGlu1a的HEK293细胞中,NAC可抑制由DHPG所引起的ERK的激活以及Bcl-2/Bax比例的下降,受体细胞表面表达没有参与NAC的这
图2 NAC对DHPG激活mGlu1a情况下ERK磷酸化,Bcl/Bax比例以及受体表面表达的影响
Figure 2 Effect of NAC on DHPG-mediated phospho-ERK, Bcl-2/Bax ratio and receptor, surface expression in mGlu1a overexpressing HEK293 cells 注: A:At 48 h or 36 h after transfection with pcDNA3.1-Flag-mGlu1aplasmid, cells were treated with 5 mmol/L NAC, with or without pretreatment with 100 μmol/L BSO, followed by DHPG (100 μmol/L) exposure. Protein levels of phospho-ERK, Bcl-2 and Bax were detected by Western blotting (left panel). Densitometric analysis was used to demonstrate changes in phospho-ERK and the Bcl-2/Bax ratio for each condition, compared with controls (left lane, right panel); B: An ELISA-based assay was used to determine alterations in the surface expression of mGlu1a. At 48 h after transfection, cells were treated with 5 mmol/L NAC for 30 min, followed by DHPG (100 μmol/L) for 5 min. Surface expression is expressed as a percentage of control cultures (left column). (1)P<0.05 and (2)P<0.01 versus control; (3)P<0.05, (4)P<0.01.
种保护作用。由此可见,mGlu1a激活所引起的细胞毒性作用区别于激动剂非依赖型的细胞凋亡。
2.3 NAC对组成型和诱导型mGlu1a所介导的过度氧化应激的作用
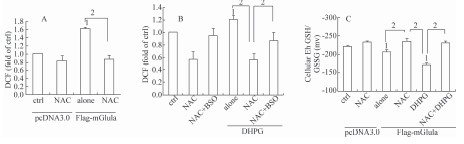
抗氧化剂NAC可以清除ROS,减轻细胞内蛋白的过度氧化[17]。因此可以推测,NAC可以通过清除细胞内ROS而缓解由mGlu1a所介导的细胞凋亡。为了验证这种假设,实验采用二氯荧光素检测方法探测细胞内ROS水平。图3A和图3B显示,NAC不仅可以减少过表达mGlu1a所产生的ROS,并且可以抑制DHPG激活mGlu1a后所引起的ROS的上升,NAC的这种抗氧化效应在BSO预处理的情况下有明显下降。这些结果显示,通过调节由mGlu1a所介导的信号通路,NAC可以降低细胞内的ROS,进而减轻细胞凋亡。
GSH是细胞内关键的抗氧化分子,它可以通过与细胞内ROS发生反应生成GSSG而缓解氧化应激。与ROS结果相一致,无论激活剂DHPG存在与否,NAC均可抑制由mGlu1a所引起的细胞内GSH/GSSG的氧化,并且BSO预处理均可逆转此结果(图3C),表明在mGlu1a的介导下,NAC可能通过调节细胞内GSH/GSSG(Eh)而减少细胞内过度的氧化应激,进而发挥调节细胞存活的效应。
图3 NAC对HEK293细胞中ROS和细胞内GSH/GSSG(Eh)水平的影响
Figure 3 Effect of NAC on level of reactive oxygen species (ROS) and cellular GSH/GSSG (Eh) 注:A:NAC scavenged ROS production caused in mGlu1aoverexpression cells. HEK293 cells were transfected with pcDNA3.0-Flag-mGlu1aor empty plasmid as a control. At 36 h after transfection, cells were treated with 5 mmol/L NAC for 9 h, and detected for cellular ROS by DCF assay. Results are expressed as fold of control (left column); B: NAC prohibited the level of ROS induced by activated group Ⅰ mGlu. At 36 h after transfection with pcDNA3.1-Flag-mGlu1aplasmid, HEK293 cells were treated with 5 mmol/L NAC, with or without pretreatment with 100 μmol/L BSO, followed by DHPG (100 μmol/L). Cells were detected for cellular ROS by DCF assay. Results are expressed as fold of control (left column); C: NAC inhibited the oxidation of cellular GSH/GSSG (Eh) induced by mGlu I. At 36 h after transfection with pcDNA3.1-Flag-mGlu1a plasmid or empty plasmid, HEK293 cells were treated with 5 mmol/L NAC for 9 h in the presence or absence of DHPG (100 μmol/L) and then were examined for GSH/GSSG (Eh).(1)P<0.05 versus control; (2)P<0.05.
3 讨论
本实验室之前结果显示,在C6,MN9D细胞以及帕金森大鼠模型中,NAC可以通过不同机制缓解mGluI介导的细胞凋亡[13],揭示在不同的细胞系和刺激情况下, NAC抑制受体介导的细胞毒性的效应不同。此文进一步探测了在过表达mGlu1a的HEK293细胞中,NAC通过调节mGlu1a所介导的激动剂依赖或非依赖通路发挥细胞保护作用,其具体机制有待进一步的探讨。瞬时转染mGlu1a所呈现的细胞毒性效应,可能与受体表达水平以及DHPG所引起的受体过度激活相关。G蛋白藕联受体(GPCR)激酶以及β-arrestins等GPRC调控蛋白决定了GPCR的调控机制及信号通路[18],其效应通过阻断G蛋白下游信号级联反应而抑制相关信号转导,同时可以通过促进GPCRs与其下游信号分子相互作用而推动受体所介导的信号转导[19]。因此在受体总量不同的情况下,GPCR可能介导了不同的信号通路。另外,Dale等[8]发现GPCR激酶也可以通过介导mGlu1a受体脱敏作用而使细胞免于凋亡。本文在过表达mGlu1a的细胞中,由于 mGluI的表达水平或激活状态不同(组成型和诱导型),NAC可能通过GPCR激酶以及β-arrestins不同地调节受体脱敏及其下游信号分子之间的相互作用,进而在细胞存活过程中对受体表面表达及受体活性发挥了不同的调控作用,最后达到保护细胞的作用。
在疾病的发生过程中,氧化应激对代谢型谷氨酸受体活性的调节作用,目前受到了广泛的关注。研究表明,氧化损伤在缺血,炎症,阿尔兹海默氏病,亨廷顿氏舞蹈病以及帕金森疾病的发病过程中具有重要的作用[20]。谷氨酸受体功能紊乱所导致的兴奋性毒性以及神经元损伤与ROS的生成密切相关[21]。同时,NAC和mGlu5拮抗剂MPEP对于帕金森疾病模型均具有一定的保护作用 。之前有研究证实,在C6,MN9D细胞以及帕金森大鼠模型中,NAC通过调节细胞内GSH/GSSG(Eh),降低细胞内的氧化应激水平,从而影响mGluI活性[13]。本文通过对ROS产生状况以及GSH/GSSG(Eh)水平的探测,发现在过表达mGlu1a的HEK293细胞中,NAC同样可以通过改变细胞内氧化应激状态而不同地调控mGlu1a介导的细胞凋亡机制。这些结果提示,在mGlu1a受到不同调控作用的情况下,由NAC所介导的细胞内氧化应激状态,可以作为影响细胞活力的刺激信号。
总之,本文在过表达mGlu1a的HEK293细胞中,NAC通过对mGlu1a以激动剂依赖和非依赖方式进行不同的活性调控,促进细胞存活并减少细胞凋亡,但是其具体机制有待进一步探究。NAC对过表达mGlu1a的调控,可能是通过清除受体激活产生的ROS,但也不排除NAC直接作用于受体上。本研究还进一步提示,在多种疾病的发展过程中,不同生理条件下mGlu1a发挥的作用较复杂,尤其是由受体表达水平增加所引起的相关效应,需要受到重视。NAC作为ROS清除剂,在这些疾病的治疗中具有非常大的应用潜力。
参考文献
[1] Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors[J]. Annu Rev Pharmacol Toxicol,1997,37: 205-237.
[2] Soloviev MM, Ciruela F, Chan WY, et al. Identification, cloning and analysis of expression of a new alternatively spliced form of the metabotropic glutamate receptor mGluR1 mRNA[J]. Biochim Biophys Acta,1999,1446(1-2):161-166.
[3] Breysse N, Amalric M, Salin, P. Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basalganglia structures in parkinsonian rats[J]. Neurosci,2003,23(23):8302-8309.
[4] Lee HG, Zhu X, O'Neill MJ, et al. The role of metabotropic glutamate receptors in Alzheimer's disease[J]. Acta Neurobiol Exp,2004,64(1):89-98.
[5] Pshenichkin S, Dolinska M, Klauzinska M, et al. Dual neurotoxic and neuroprotective role of metabotropic glutamate receptor 1 in conditions of trophic deprivationpossible role as a dependence receptor[J]. Neuropharmacology,2008,55(4):500-508.
[6] Allen JW, Knoblach SM, Faden AI. Activation of Group Ⅰ metabotropic glutamate receptors reduces neuronal apoptosis but increases necrotic cell death in vitro[J]. Cell Death Differ,2000,7(5):470-476.
[7] Deng WB, Wang H, Rosenberg PA, et al. Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress[J]. Proc Natl Acad Sci USA,2004,101(20):7751-7756.
[8] Dale LB, Bhattacharya M, Anborgh PH, et al. G protein coupled receptor kinasemediated desensitization of metabotropic glutamate receptor 1A protects against cell death[J]. Biol Chem,2000,275(49):38213-38220.
[9] Rasola A, Sciacovelli M, Chiara F, et al. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition[J]. Proc Natl Sci USA,2010,107(2):726-731.
[10] Chen XH, Lan XJ, Mo SL, et al. p38 and ERK, but not INK, are involved in copperinduced apoptosis in cultured cerebellar granule neurons[J]. Biochem Bioph Res Commun,2009,379(4):944-948.
[11] Meli E, Baronti R, Pangallo M, et al. Group Ⅰ metabotropic glutamate receptors stimulate the activity of poly(ADPribose) polymerase in mammalian mGlu1transfected cells and in cortical cell cultures[J]. Neuropharmacology,2005,49(Suppl 1):80-88.
[12] Francesconi A, Kumari R, Zukin RS. Regulation of group Ⅰ metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway[J].Neurosci,2009,29(11):3590-3602.
[13] Sun LL, Gu L, Wang ST, et al. Nacetylcysteine protects against apoptosis through modulation of group Ⅰ metabotropic glutamate receptor activity[J]. PLoS One,2012,7(3):e32503.
[14] Lee JH, Lee J, Choi KY, et al. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5[J]. Proc Natl Acad Sci USA,2008,105(34):12575-12580.
[15] Doherty AJ, Coutinho V, Collingridge GL, et al. Rapid internalization and surface expression of a functional, fluorescently tagged Gproteincoupled glutamate receptor[J]. Biochem,1999,341(Pt 2):415-422.
[16] Page G, Khidir FAL, Pain S, et al. Group Ⅰ metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin(mTOR) and extracellular signalregulated kinase(ERK 1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes[J]. Neurochem Int,2006,49(4):413-421.
[17] Martin V, Herrera F, GarciaSantos G, et al. Signaling pathways involved in antioxidant control of glioma cell proliferation[J]. Free Radic Biol Med,2007,42(11):1715-1722.
[18] Schmid CL, Raehal KM, Bohn LM. Agonistdirected signaling of the serotonin 2A receptor depends on betaarrestin2 interactions in vivo[J]. Proc Natl Acad Sci USA,2008,105(3):1079-1084.
[19] Schmid CL, Bohn LM. Physiological and pharmacological implications of betaarrestin regulation[J]. Pharmacol Ther,2009,121(3):285-293.
[20] Uttara B, Singh AV, Zamboni P, et al. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options[J]. Curr Neuropharmacology,2009,7(1):65-74.
[21] Sun XL, Zeng XN, Zhou F, et al. KATP channel openers facilitate glutamate uptake by GluTs in rat primary cultured Astrocytes[J]. Neuropharmacology,2008,28(6):757.
[22] Munoz AM, Rey P, SotoOtero R, et al. Systemic administration of Nacetylcysteine protects dopaminergic neurons against 6hydroxydopamineinduced degeneration[J]. Neurosci Res,2004,76(4):551-562.
[23] Morin N, Gregoire L, GomezMancilla B, et al. Effect of the metabotropic glutamate receptor type 5 antagonists MPEP and MTEP in parkinsonian monkeys[J]. Neuropharmacology,2010,58(7):981-986.
[2] Soloviev MM, Ciruela F, Chan WY, et al. Identification, cloning and analysis of expression of a new alternatively spliced form of the metabotropic glutamate receptor mGluR1 mRNA[J]. Biochim Biophys Acta,1999,1446(1-2):161-166.
[3] Breysse N, Amalric M, Salin, P. Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basalganglia structures in parkinsonian rats[J]. Neurosci,2003,23(23):8302-8309.
[4] Lee HG, Zhu X, O'Neill MJ, et al. The role of metabotropic glutamate receptors in Alzheimer's disease[J]. Acta Neurobiol Exp,2004,64(1):89-98.
[5] Pshenichkin S, Dolinska M, Klauzinska M, et al. Dual neurotoxic and neuroprotective role of metabotropic glutamate receptor 1 in conditions of trophic deprivationpossible role as a dependence receptor[J]. Neuropharmacology,2008,55(4):500-508.
[6] Allen JW, Knoblach SM, Faden AI. Activation of Group Ⅰ metabotropic glutamate receptors reduces neuronal apoptosis but increases necrotic cell death in vitro[J]. Cell Death Differ,2000,7(5):470-476.
[7] Deng WB, Wang H, Rosenberg PA, et al. Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress[J]. Proc Natl Acad Sci USA,2004,101(20):7751-7756.
[8] Dale LB, Bhattacharya M, Anborgh PH, et al. G protein coupled receptor kinasemediated desensitization of metabotropic glutamate receptor 1A protects against cell death[J]. Biol Chem,2000,275(49):38213-38220.
[9] Rasola A, Sciacovelli M, Chiara F, et al. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition[J]. Proc Natl Sci USA,2010,107(2):726-731.
[10] Chen XH, Lan XJ, Mo SL, et al. p38 and ERK, but not INK, are involved in copperinduced apoptosis in cultured cerebellar granule neurons[J]. Biochem Bioph Res Commun,2009,379(4):944-948.
[11] Meli E, Baronti R, Pangallo M, et al. Group Ⅰ metabotropic glutamate receptors stimulate the activity of poly(ADPribose) polymerase in mammalian mGlu1transfected cells and in cortical cell cultures[J]. Neuropharmacology,2005,49(Suppl 1):80-88.
[12] Francesconi A, Kumari R, Zukin RS. Regulation of group Ⅰ metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway[J].Neurosci,2009,29(11):3590-3602.
[13] Sun LL, Gu L, Wang ST, et al. Nacetylcysteine protects against apoptosis through modulation of group Ⅰ metabotropic glutamate receptor activity[J]. PLoS One,2012,7(3):e32503.
[14] Lee JH, Lee J, Choi KY, et al. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5[J]. Proc Natl Acad Sci USA,2008,105(34):12575-12580.
[15] Doherty AJ, Coutinho V, Collingridge GL, et al. Rapid internalization and surface expression of a functional, fluorescently tagged Gproteincoupled glutamate receptor[J]. Biochem,1999,341(Pt 2):415-422.
[16] Page G, Khidir FAL, Pain S, et al. Group Ⅰ metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin(mTOR) and extracellular signalregulated kinase(ERK 1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes[J]. Neurochem Int,2006,49(4):413-421.
[17] Martin V, Herrera F, GarciaSantos G, et al. Signaling pathways involved in antioxidant control of glioma cell proliferation[J]. Free Radic Biol Med,2007,42(11):1715-1722.
[18] Schmid CL, Raehal KM, Bohn LM. Agonistdirected signaling of the serotonin 2A receptor depends on betaarrestin2 interactions in vivo[J]. Proc Natl Acad Sci USA,2008,105(3):1079-1084.
[19] Schmid CL, Bohn LM. Physiological and pharmacological implications of betaarrestin regulation[J]. Pharmacol Ther,2009,121(3):285-293.
[20] Uttara B, Singh AV, Zamboni P, et al. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options[J]. Curr Neuropharmacology,2009,7(1):65-74.
[21] Sun XL, Zeng XN, Zhou F, et al. KATP channel openers facilitate glutamate uptake by GluTs in rat primary cultured Astrocytes[J]. Neuropharmacology,2008,28(6):757.
[22] Munoz AM, Rey P, SotoOtero R, et al. Systemic administration of Nacetylcysteine protects dopaminergic neurons against 6hydroxydopamineinduced degeneration[J]. Neurosci Res,2004,76(4):551-562.
[23] Morin N, Gregoire L, GomezMancilla B, et al. Effect of the metabotropic glutamate receptor type 5 antagonists MPEP and MTEP in parkinsonian monkeys[J]. Neuropharmacology,2010,58(7):981-986.
|
扩展功能
|
|
| 本文信息 | |
| PDF全文 | |
| HTML全文 | |
| 参考文献 | |
| 服务与反馈 | |
| 加入引用管理器 | |
| 引用本文 | |
| Email Alert | |
| 本文作者相关文章 | |
| 王舒婷 | |
| 夏宁 | |
| 袁记方 | |
| 张红 | |
| PubMed | |
| Article by WANG Shu-ting | |
| Article by XIA Ning | |
| Article by YUAN Ji-fang | |
| Article by ZHANG Hong | |