扩展功能
文章信息
- 谭红丽, 程雪琴, 王勇, 黄艳梅, 刘炜, 张丽娟
- TAN Hong-li, CHENG Xue-qin, WANG Yong, HUANG Yan-mei, LIU Wei, ZHANG Li-juan
- 多耐鲍曼不动杆菌分子流行病学分析
- Molecular characterization of multi-drug-resistant Acinetobacter baumannii isolated from patients in a hospital in Yunnan
- 疾病监测, 2015, 30(1): 8-13
- Disease Surveillance, 2015, 30(1): 8-13
- 10.3784/j.issn.1003-9961.2015.01.004
-
文章历史
- 收稿日期:2014-07-25
2. 中国疾病预防控制中心传染病预防控制所, 北京 102206;
3. 新疆石河子大学, 新疆 石河子 832003
2. Institute for Communicable Disease Prevention and Control, Chinese Center for Disease Control and Prevention, Beijing 102206, China;
3. College of Animal Science & Technology, Shihezi University, Shihezi 832003, Xinjiang, China
鲍曼不动杆菌(Acinetobacter baumannii)院内感染是当前全球范围内面临的巨大挑战。该菌的耐药尤其是多耐、泛耐菌株的出现与快速播散给临床治疗带来极大困难[1]。2000年以来,云南省第三人民医院检验科发现,多重耐药鲍曼不动杆菌分离率不断增加。临床及流行病学调查发现,菌株分离患者均符合院内感染(health care-associated,HA)标准[2]。为分析该菌分子流行病学特征、预防控制院内感染暴发流行,中国疾病预防控制中心(CDC)传染病预防控制所与云南省第三人民医院合作进行了鲍曼不动杆菌耐药表型及耐药基因分子流行病学分析。
1 材料与方法 1.1 菌株2013年69月,云南省第三人民医院入住重症监护室(ICU)48 h后院内感染患者分离的鲍曼不动杆菌28株。其中,22株分离于痰标本、4株来自尿标本、2株来自分泌物。平均年龄70.4岁(27.0~93.0岁)。男女性别比为6∶1(24/4)。患者均来自全省范围内到昆明就诊住院患者。
1.2 菌株鉴定及药物敏感性鉴定法国梅里埃全自动微生物分析仪(bioMerieux VITEK-2 system)进行菌株鉴定及药物敏感分析。细菌鉴定卡及药敏卡均为法国梅里埃公司提供。菌种鉴定进一步采用原核细菌16 SrRNA基因扩增及测序分析完成[3]。多耐菌(multi-drug-resistant,MDR)、广泛耐药菌(extensively drug-resistant,XDR)及泛耐药菌(pandrug-resistant,PDR)等按国际标准分类[4]。
1.3 耐药基因分子流行病学分析聚合酶链反应(PCR)扩增包括超广谱β-内酰胺酶bla基因、氟喹诺酮类及氨基糖苷类抗生素耐药基因、IS插入元件及整合子等。扩增引物及反应条件见表 1参考文献。引物由北京赛百盛生物技术有限公司合成。细菌质粒提取用百泰克生物技术有限公司试剂盒(Cat No.DP1002)。DNA提取用德国 QIAGEN试剂(Cat No. 69506)。PCR产物送北京擎科生物技术有限公司双向测序,采用NCBI网站Blast平台进行序列同源比较。
| 目标基因 | 引物名称 | 引物序列(5′~3′) | 片段大小(bp) | 参考文献 |
| β-内酰胺类 | ||||
| TEM | TEM-F | TCC GCT CAT GAG ACA ATA ACC | 931 | [5] |
| TEM-R | TTG GTC TGA CAG TTA CCA ATG C | |||
| SHV | SHV-F | TGG TTA TGC GTT ATA TTC GCC | 868 | [5] |
| SHV-R | GGT TAG CGT TGC CAG TGC T | |||
| CTX-M | CTX-F | TCT TCC AGA ATA AGG AAT CCC | 909 | [5] |
| CTX-R | CCG TTT CCG CTA TTA CAA AC | |||
| VEB | VEB-F1 | GAT AGG AGT ACA GAC ATA TG | 914 | [5] |
| VEB-R1 | TTT ATT CAA ATA GTA ATT CCA CG | |||
| PER | PER-F | ATG AAT GTC ATC ACA AAA TG | 927 | [5] |
| PER-R | TCA ATC CGG ACT CAC T | |||
| GES | GES-F | ATG CGC TTC ATT CAC GCA C | 864 | [5] |
| GES-R | CTA TTT GTC CGT GCT CAG G | |||
| KPC | KPC-F | ATG TCA CTG TAT CGC CGT CT | 882 | [6] |
| KPC-R | TTT TCA GAG CCT TAC TGC | |||
| NDM | NDM-F | GGT TTG GCG ATC TGG TTT TC | 621 | [6] |
| NDM-R | CGG AAT GGC TCA TCA CGA | |||
| IMP | IMP-F | GGA ATA GAG TGG CTT AAT TCT C | 624 | [6] |
| IMP-R | CCA AAC CAC TAC GTT ATC | |||
| VIM | VIM-F | GGT CTC ATT GTC CGT GAT GGT GAT GAG | 271 | [6] |
| VIM-R | CTC GAT GAG AGT CCT TCT AGA G | |||
| CMY | CMY-F | TGG CCA GAA CTG ACA GGC AAA | 462 | [6] |
| CMY-R | TTT CTC CTG AAC GTG GCT GGC | |||
| DHA | DHA-F | AAC TTT CAC AGG TGT GCT GGG T | 405 | [6] |
| DHA-R | CCG TAC GCA TAC TGG CTT TGC | |||
| FOX | FOX-F | AAC ATG GGG TAT CAG GGA GAT G | 190 | [6] |
| FOX-R | CAA AGC GCG TAA CCG GAT TGG | |||
| OXA | OXA-2-F | AAG AAA CGC TAC TCG CCT GC | 478 | [5] |
| OXA-2-R | CCA CTC AAC CCA TCC TAC CC | |||
| OXA-10-F | GTC TTT CGA GTA CGG CAT TA | 720 | [5] | |
| OXA-10-R | ATT TTC TTA GCG GCA ACT TAC | |||
| OXA-23-F | GAT CGG ATT GGA GAA CCA GA | [7] | ||
| OXA-23-R | ATT TCT GAC CGC ATT TCC AT | |||
| OXA-24-F | GGT TAG TTG GCC CCC TTA AA | [7] | ||
| OXA-24-R | AGT TGA GCG AAA AGG GGA TT | |||
| OXA-51-F | TAA TGC TTT GAT CGG CCT TG | [7] | ||
| OXA-51-R | TGG ATT GCA CTT CAT CTT GG | |||
| OXA-48-F | TTG GTG GCA TCG ATT ATC GG | 743 | [7] | |
| OXA-48-R | GAG CAC TTC TTT TGT GAT GGC | |||
| OXA-58-F | AAG TAT TGG GGC TTG TGC TG | [7] | ||
| OXA-58-R | CCC CTC TGC GCT CTA CAT AC | |||
| 氟喹诺酮类 | ||||
| qnrA | qnrA-F | ATT TCT CAC GCC AGG ATT TG | 627 | [6] |
| qnrA-R | GAT CGG CAA AGG TTA GGT CA | |||
| qnrB | qnrB-F | GAT CGT GAA AGC CAG AAA GG | 469 | [6] |
| qnrB-R | ACG ATG CCT GGT AGT TGT CC | |||
| qnrC | qnrC-F | GGG TTG TAC ATT TAT TGA ATC G | 307 | [6] |
| qnrC-R | CAC CTA CCC ATT TAT TTT CA | |||
| qnrD | qnrD-F | CGA GAT CAA TTTA CGG GGA ATA | 533 | [6] |
| qnrD-R | AAC AAG CTG AAG CGC CTG 533 | |||
| qnrS | qnrS-F | ACG ACA TTC GTC AAC TGC AA | 417 | [6] |
| qnrS-R | TAA ATT GGC ACC CTG TAG GC | |||
| aac(6′) -Ib-cr | aac(6′) -Ib-cr -F | TTG CGA TGC TCT ATG AGT GGC TA | 482 | [6] |
| aac(6′) -Ib-cr -R | CTC GAA TGC CTG GCG TGT TT | |||
| qepA | qepA-F | AAC TGC TTG AGC CCG TAG AT | 596 | [6] |
| qepA-R | GTC TAC GCC ATG GAC CTC AC | |||
| gyrA突变 | gyrA-F | CGA CCT TGC GAG AGA AAT | 626 | [6] |
| gyrA-R | GTT CCA TCA GCC CTT CAA | |||
| 氨基糖苷类 | ||||
| aacA4 | aacA4-F | ATG ACT GAG CAT GAC CTT GCG | 540 | [6] |
| aacA4-R | TTA GGC ATC ACT GCG TGT TCG | |||
| aacC1 | aacC1-F | ATG GGC ATC ATT CGC ACA TGT AGG | 873 | [6] |
| aacC1-R | TTA GGT GGC GGT ACT TGG GTC | |||
| aacC2 | aacC2-F | ATG CAT ACG CGG AAG GCA ATA AC | 861 | [6] |
| aacC2-R | CTA ACC GGA AGG CTC GCA AG | |||
| aadA1 | aadA1-F | ATG AGG GAA GCG GTG ATC G | 792 | [6] |
| aadA1-R | TTA TTT GCC GAC TAC CTT GGT G | |||
| aadB | aadB-F | ATG GAC ACA ACG CAG GTC GC | 534 | [6] |
| aadB-R | TTA GGC CGC ATA TCG CGA CC | |||
| aphA6 | aphA6-F | ATG GAA TTG CCC AAT ATT ATT C | 781 | [6] |
| aphA6-R | TCA ATT CAA TTC ATC AAG TTT TA | |||
| 插入元件/整合子 | ||||
| int1 | int 1F | CCT CCC GCA CGA TGA TC | 280 | [8] |
| int 1R | TCC ACG CAT CGT CAG GC | |||
| ISCR1 | CR1F | ATG TCT CTG GCA AGG AAC GC | 1450 | [8] |
| CR1R | AGA CGA CTC TGT GAT GGA TC | |||
| ISEcp1 | IS-F | GTG CCC AAG GGG AGT GTA TG | 615 | [5] |
| IS-R | ACY TTA CTG GTR CTG CAC AT |
为分析遗传变异关系,试验菌株进行了MLST及PFGE分型。MLST引物、操作及MLST 序列型(STs)分析见www.pasteur.fr/mlst网站。MLST分型使用7个基因包括cpn60、fusA、gltA、pyrG、recA、rplB、rpoB。PFGE采用CHEF Mapper XA脉冲场电泳仪(Bio-Rad Laboratories,Hercules,CA)按参考方法进行[9],PFGE带型分析使用Fingerprinting Ⅱ软件(version 3.0,Bio-Rad Laboratories,Hercules,CA),相似性大于80%归为一簇。
1.5 统计学分析应用SPSS 软件(version 9.1,SAS Institute,Inc.,Cary,NC) 进行统计分析。变量间比较用χ2检验。P<0.05为差异有统计学意义。
2 结果 2.1 耐药分析细菌耐药结果见表 2。本次检测的鲍曼不动杆菌按耐药分类标准,78.6%(22/28)菌株为MDR,14.3%(4/28)菌株为广泛耐药菌(XDR),7.1%为泛耐药菌(PDR)。100%菌株不仅对一代/二代头孢类抗生素耐药,且对三代/四代抗生素CRO、CAZ、 FEP及单环β-内酰胺类(ATM)、氟喹诺酮类(CIP、LEV)、氨基糖苷类(AN)及磺胺类(FD)等耐药。值得注意的是92.9%菌株对碳青霉烯类抗生素耐药。
| 注:(1)S:敏感菌株; I:中介耐药菌株; R:耐药菌株。 | ||||
| 分类 | 抗生素 名称 | 细菌耐药及敏感率(%)(1) | ||
| S | I | R | ||
| 氨苄西林 | AMP | 0.0 | 0.0 | 100.0 |
| 羧苄青霉素+ β-内酰胺抑制剂 | TZP | 0.0 | 7.1 | 92.9 |
| 青霉素+β-内酰胺抑制剂 | SAM | 7.1 | 50.0 | 42.9 |
| 一代/二代头孢霉素 | CXM | 0.0 | 0.0 | 100.0 |
| CFZ | 0.0 | 0.0 | 100.0 | |
| 三代/四代头孢霉素 | CRO | 0.0 | 0.0 | 100.0 |
| CAZ | 0.0 | 0.0 | 100.0 | |
| SCF | 35.7 | 57.1 | 7.1 | |
| FEP | 0.0 | 0.0 | 100.0 | |
| 头孢霉素 | CTT | 0.0 | 14.3 | 85.7 |
| 碳青霉烯类 | ETP | 7.1 | 0.0 | 92.9 |
| MEM | 7.1 | 0.0 | 92.9 | |
| IMP | 7.1 | 0.0 | 92.9 | |
| 单环β-内酰胺类 | ATM | 0.0 | 0.0 | 100.0 |
| 氟喹诺酮类 | CIP | 0.0 | 0.0 | 100.0 |
| LEV | 0.0 | 0.0 | 100.0 | |
| 氨基糖苷类 | GM | 7.1 | 0.0 | 92.9 |
| TM | 7.1 | 14.3 | 78.6 | |
| AN | 0.0 | 0.0 | 100.0 | |
| 叶酸代谢途径抑制剂 | SXT | 14.3 | 0.0 | 85.7 |
| 磺胺类 | FD | 0.0 | 0.0 | 100.0 |
以质粒及基因组DNA模板,PCR扩增及序列分析显示,除了blaOXA 基因外,其他β-内酰胺类耐药基因(表 2)均为阴性。在质粒介导的blaOXA 阳性菌株中,64.3%(18/28)菌株为复合携带blaOXA-2/23/48/51/58基因。7.1%(2/28) 菌株携带bla OXA-2 /-48/-51/-58基因 ,7.1%(2/28) 菌株携带blaOXA-2 /-23/-48/-51基因 ,7.1%(2/28) 菌株携带bla OXA-2 /-23/-51基因。14.3%(4/28) 菌株单一携带blaOXA-51 基因。7种氟喹诺酮类抗性基因检测发现,100%菌株携带 aac(6’)-Ib-cr,85.7%菌株携带qnrA,78.6%菌株携带 qnrD。其他4种基因包括qnrB、qnrC、qnrS和qepA没有检测到,也没有检测到gyrA突变。6种氨基糖苷类抗性基因检测发现,100%菌株携带aacA4,85.7%菌株携带aadA1,7.1%菌株携带aacC2,没有检测到aacC1、aadB 和aphA6。移动元件及整合子检测发现100%菌株携带int1,85.7%菌株携带ISCR1。没有检测到ISEcp1。
2.3 MLST及PFGE分型特征使用7个保守基因包括cpn60、fusA、gltA、pyrG、recA、rplB、rpoB 进行MLST分型,结果提示,同欧洲地区流行株分布极为相似,ST2为优势序列型,占85.7%。与GenBank收录的12个全基因组比较并进化分析,结果本试验菌株与我国天津鲍曼不动杆菌MDR-TJ关系密切[10]。此外还检测到ST5及ST6,分别占7.1%。PFGE结果显示所有分型成功菌株按聚类分析标准(>80%相似性为一群)为同一克隆群,见图 1。
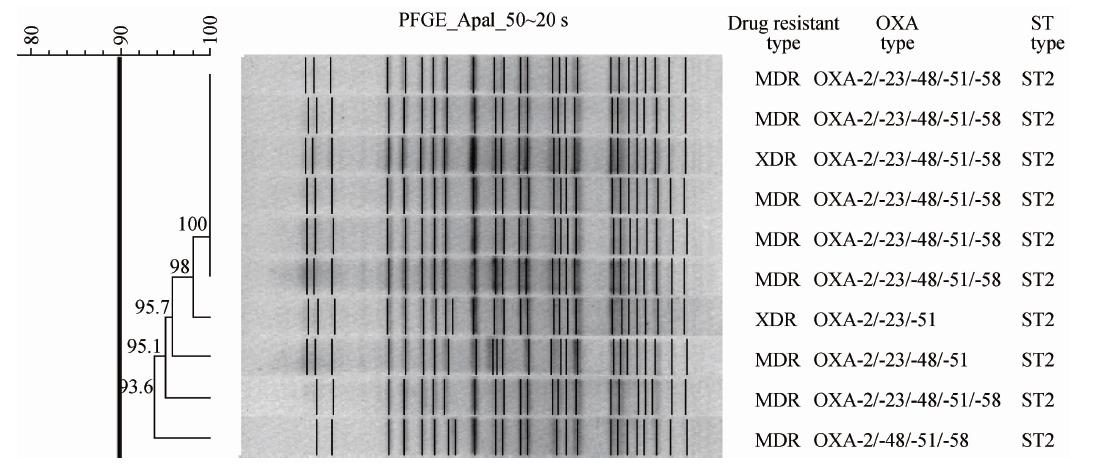
|
| 图 1 代表性菌株PFGE聚类分析 Fig. 1 PFGE pattern and drug-resistant genotype of isolates |
多耐鲍曼不动杆菌院内感染暴发已经成为当今世界重大的公共卫生问题,尤其是临床ICU重症患者感染后病死率高达30%[11]。本研究实验菌株来自同一医院不同ICU病房,均符合院内感染标准并证实全部菌株呈现多重耐药,尤其是92.9%菌株对3种试验碳青霉烯类抗生素全部耐药。除MDR菌株(78.6%)外,还出现了XDR(14.3%)及PDR(7.1%)菌株。特别值得注意的是这些不同ICU病房的高度耐药菌株遗传进化关系属于同一PFGE克隆群(图 1),提示当地医院可能存在有碳青霉烯酶多耐鲍曼不动 杆菌院内感染流行,有待深入流行学及临床调查。ST2序列型为欧洲地区主要流行株,在意大利、希腊频繁暴发院内感染事件[12,13],近年在亚洲中东地区及北非地区也时有报道[14,15,16]。这一分子流行病学结果进一步为临床院内感染提供了分子遗传学证据。流行株耐药基因主要包括质粒介导的D类β-内酰胺类抗性基因-OXA、氟喹诺酮类[aac(6’)-Ib-cr、 qnrA 及qnrD]以及氨基糖苷类(aacA4、 aadA1及 aacC2)。D类β-内酰胺类抗性基因是近年发现并呈全球分布,该类基因不仅水解广谱头孢菌素,而且与碳青霉烯类抗生素抗性密切相关。最新研究证实,世界范围内有8群blaOXA基因[17]。本研究全部菌株携带该类基因,并表现高度复合携带状态(图 1)。100%菌株携带blaOXA-51,85.7%菌株复合携带多种blaOXA基因,blaOXA-2/23/48/51/58复合携带菌株占64.3%。除了世界范围内广泛分布的blaOXA-23[1],78.6%菌株还检测到最新发现的blaOXA-48基因。我国流行株主要携带blaOXA-23基因 [18,19,20],近年blaOXA-51和blaOXA-58也屡见报道[21,22],并且存在blaOXA-23 和其他blaOXA基因复合携带状况[21,23,24]。本试验菌株不仅复合携带多种blaOXA基因,而且大多数菌株还复合携带氟喹诺酮类及氨基糖苷类多种基因。
本试验100%菌株携带整合酶基因int 1,85.7%菌株携带ISCR1。这一分子遗传学结果与耐药表型高度一致,也进一步提示当地细菌多重耐药及快速传播扩散与这些基因捕获元件密切相关[25]。 ISEcp1插入元件位于blaCTX-M基因上游,发挥启动子作用。本试验没有检测到blaCTX-M基因及相关的ISEcp1插入元件。
本研究结果提示当地医院ST2型多重耐药产碳青霉烯酶鲍曼不动杆菌正在流行,对ICU重症患者治疗构成严重威胁,进一步开展详细的流行病学调查和采取必要的防控措施及合理应用抗生素是当务之急。
| [1] | Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii:Emergence of a successful pathogen[J]. Clin Micrbio Rev,2008,21(3):538-582. |
| [2] | Apisarnthanarak A, Kiratisin P, Saifon P, et al. Risk factors for and outcomes of healthcare-associated infection due to extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella pneumoniae in Thailand[J]. Infect Control Hosp Epidemiol,2007,28(7):873-876. |
| [3] | Weisburg WG, Barns SM, Peleetier DA, et al. 16S ribosomal DNA amplification for phylogenetic study[J]. J Bacteriol,1991,173(2):697-703. |
| [4] | Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria:an international expert proposal for interim standard definitions for acquired resistance[J]. Clin Microbiol Infect,2012,18(3):268-281. |
| [5] | Kiratisin P, Apisarnthanarak A, Laesripa C, et al. Molecular characterization and epidemiology of extended-spectrum-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates causing health care-associated infection in Thailand, where the CTX-M family is endemic[J]. Antimicrob Agents Chemother,2008,52(8):2818-2824. |
| [6] | Li B, Yi Y, Wang Q, et al. Analysis of drug resistance determinants in Klebsiella pneumoniae isolates from a tertiary-care hospital in Beijing, China[J]. PLoS One,2012,7:e42280. |
| [7] | Woodford N, Ellington MJ, Coelho JM, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp[J]. Int J Antimicrob Agents,2006,27(4), 351-353. |
| [8] | Chen X, Yuan M, Li GX, et al. Dissemination of int1 gene and ISCR1 and their relations with multi-drug resistance in clinical isolates[J]. Chin J Znoonoses,2013,29(7):646-652. |
| [9] | Mugnier PD, Poirel L, Naas T, et al. Worldwide dissemination of the blaOXA-23 carbapenemase gene of Acinetobacter baumannii[J]. Emerg Infect Dis,2010,16(1):35-40. |
| [10] | Huang H, Yang ZL, Wu XM,et al. Complete genome sequence of Acinetobacter baumannii MDR-TJ and insights into its mechanism of antibiotic resistance[J].J Antimicrob Chemother,2012,67(12):2825-2832. |
| [11] | Wilson SJ, Knipe CJ, Zieger MJ, et al. Direct costs of multidrug-resistant Acinetobacter baumannii in the burn unit of a public teaching hospital[J]. Am J Infect Control,2004,32(6):342-344. |
| [12] | Agodi A, Voulgari E, Barchitta M,et al. Spread of a carbapenem-and colistin-resistant Acinetobacter baumannii ST2 clonal strain causing outbreaks in two Sicilian hospitals[J]. J Hosp Infect,2014,86(4):260-266. |
| [13] | Villar M, Cano ME, Gato E,et al. Epidemiologic and clinical impact of Acinetobacter baumannii colonization and infection:a reappraisal[J].Medicine (Baltimore),2014,93(5):202-210. |
| [14] | Bakour S, Alsharapy SA, Touati A,et al. Characterization of Acinetobacter baumannii clinical isolates carrying blaOXA-23 carbapenemase and 16S rRNA methylase armA genes in Yemen[J]. Microb Drug Resist,2014,[Epub ahead of print]. |
| [15] | Hojabri Z, Pajand O, Bonura C,et al. Molecular epidemiology of Acinetobacter baumannii in Iran:endemic and epidemic spread of multiresistant isolates[J]. J Antimicrob Chemother,2014,69(9):2383-2387. |
| [16] | Bakour S, Touati A, Bachiri T,et al. First report of 16S rRNA methylase ArmA-producing Acinetobacter baumannii and rapid spread of metallo-β-lactamase NDM-1 in Algerian hospitals[J]. J Infect Chemother,2014,[Epub ahead of print]. |
| [17] | Walther-Rasmussen J,Hoiby N. OXA-type carbapenemases[J]. J Antimicrob Chemother,2006,57(3):373-383. |
| [18] | Zhao WS, Liu GY, Mi ZH,et al. Coexistence of blaOXA-23 with armA and novel gyrA mutation in a pandrug-resistant Acinetobacter baumannii isolate from the blood of a patient with haematological disease in China[J]. J Hosp Infect,2011,77(3):278-279. |
| [19] | Dai XT, Sun FJ, Chen ZH,et al. The Epidemiology and resistance mechanisms of Acinetobacter baumannii isolates from the respiratory department ICU of a hospital in China[J].Microb Drug Resist,2014,[Epub ahead of print]. |
| [20] | He C, Xie Y, Zhang L, et al. Increasing imipenem resistance and dissemination of the ISAba1-associated blaOXA-23 gene among Acinetobacter baumannii isolates in an intensive care unit[J].J Med Microbiol,2011,60(Pt 3):337-341. |
| [21] | Ho CM, Ho MW, Chi CY,et al. Repeated colonization by multi-drug-resistant Acinetobacter calcoaceticus-A. baumannii complex and changes in antimicrobial susceptibilities in surgical intensive care units[J]. Surg Infect (Larchmt),2013,14(1):43-48. |
| [22] | Fu Y, Jiang J, Zhou H, et al. Characterization of a novel plasmid type and various genetic contexts of blaOXA-58 in Acinetobacter spp. from multiple cities in China[J].PLoS One,2014,9(1):e84680. |
| [23] | Zhou H, Pi BR, Yang Q, et al. Dissemination of imipenem-resistant Acinetobacter baumannii strains carrying the ISAba1blaOXA-23 genes in a Chinese hospital[J]. J Med Microbiol,2007,56(Pt 8):1076-1080. |
| [24] | Zhou H, Du XX, Yang Q,et al. Study on carbapenemase and 16S rRNA methylase of imipenem-resistant Acinetobacter baumannii[J].Chin J Epidemiol,2009,30(3):269-272. |
| [25] | Marque S, Poirel L, Heritier C, et al. Regional occurrence of plasmid mediated carbapenem-hydrolyzing oxacillinase OXA-58 in Acinetobacter spp. in Europe[J]. J Clin Microbiol,2005,43(9):4885-4888. |
 2015, Vol. 30
2015, Vol. 30


