扩展功能
文章信息
- 王欣儒, 王建平, 罗霞, 孙强正
- WANG Xin-ru, WANG Jian-ping, LUO Xia, SUN Qiang-zheng
- 福氏志贺菌gtrI基因突变导致的血清型回复转换
- Serotype conversion due to gtrI gene dysfunctional mutation in Shigella flexneri serotype Y strains
- 疾病监测, 2015, 30(12): 1024-1027
- Disease Surveillance, 2015, 30(12): 1024-1027
- 10.3784/j.issn.1003-9961.2015.12.009
-
文章历史
- 收稿日期: 2015-03-13
2. 传染病预防控制国家重点实验室, 中国疾病预防控制中心传染病预防控制所, 北京 102206
2. State Key Laboratory for Communicable Disease Prevention and Control, National Institute for Communicable Disease Prevention and Control, Chinese Center for Disease Control and Prevention, Beijing 102206, China
福氏志贺菌是发展中国家细菌性痢疾的重要病原菌,全球每年有1.25亿例痢疾病例,其中由福氏志贺菌引起的占80%以上[1]。根据O抗结构的差异,福氏志贺菌最初分为13种血清型(1a、1b、2a、2b、3a、3b、4a、4b、5a、5b、6、X和Y)。近几年来,一新的O抗修饰方式及其介导的血清型陆续被揭示和发现,进一步将1a、2a、3a、4a、5a、X和Y分为不同的亚型,目前的血清型达30余种[2]。除血清6型外,所有血清型的O抗具有相同的四糖骨架,在骨架的不同糖基上进行乙酰化、糖基化和磷酸乙醇胺化修饰,产生了不同的抗原表位,形成了不同的血清型[3, 4, 5, 6, 7]。Y血清型具有基本的O抗四糖骨架,没有任何修饰。乙酰化修饰和磷酸乙醇胺化修饰是由单个基因oac(oacA、oacB、oacC、oacD)和opt (optII和optIII)介导完成的,他们分别位于基因组和质粒上[4, 5, 7, 8, 9];而糖基化修饰是由血清型转换噬菌体携带的、由3个基因形成的gtr基因簇(gtrA、gtrB、gtrtype)介导完成的,前两个基因高度保守,而第三个基因gtrtype编码糖基转移酶,是血清型特异的[10]。
通过噬菌体、质粒等可移动元件携带的血清型O抗修饰基因在不同血清型菌株间的转移,介导血清型转换,这是福氏志贺菌血清型产生机制。gtrI基因簇(gtrA、gtrB、gtrI)是由血清型转换噬菌体SfI携带,通过感染Y、3b和X血清型菌株,介导在四糖骨架的N-乙酰葡糖胺基团添加糖基,呈现抗原表位I,血清型分别转换为1a、1b 和1d [11]。其中1a血清型O抗四糖骨架仅在N-乙酰葡糖胺基团发生糖基化修饰,而1b和1d血清型O抗四糖骨架除N-乙酰葡糖胺基团上的糖基化修饰外,还分别由噬菌体Sf6和SfX介导在第一个鼠李糖和第三个鼠李糖上发生乙酰化和糖基化修饰,导致群抗原表位6、7、8的出现[8]。
人体对志贺菌的免疫是血清型特异的,即人体只对同样血清型的再次感染具有免疫保护作用。因此,O抗修饰基因的功能性失活突变,同噬菌体、质粒结构介导的O抗修饰和血清型转换一样,是菌体与人类斗争过程中获得适用性进化。为进一步丰富对福氏志贺菌血清型转换及其机制的认识,本研究通过对监测收集的福氏志贺菌菌株,进行血清型多重聚合酶链反应(PCR)分型研究。
1 材料与方法 1.1 菌株和培养条件5株血清型Y福氏志贺菌株从19972010年间全国细菌性痢疾监测收集菌株中筛选出(表 1)。菌株2001019(1a)和2003036(Y)作为对照阳性。志贺菌在LB琼脂平板上培养,挑取单菌落进行血清学鉴定。血清型鉴定使用日本生研公司志贺菌抗血清(Denka Seiken,Japan)和自制抗原表位MASF IV-1抗血清[12],采用经典的玻片凝集法。
1.2 主要试剂及材料TaqDNA聚合酶、PfuDNA聚合酶购自TaKaRa公司;多重PCR扩增试剂盒购自Qiagen公司;DNA回收纯化试剂盒为QIAGEN公司产品;染色体DNA提取试剂盒购自上海生工公司;其他试剂均为分析纯。
1.3 血清型多重PCR鉴定福氏志贺菌血清型多重PCR引物及其体系检测按照参考文献[13]进行。PCR条件为:94 ℃预变性15 min ;94 ℃变性30 s ,55℃退火30 s ,72℃延伸60 s ,共30 个循环;最后72 ℃延伸10 min。
1.4 PCR扩增产物纯化、测序及序列比对根据gtrI基因簇序列设计引物gtrIAB-U:TTA TTC ATT CGT TAG TGT GGT T ;gtrIAB-L:CCT TAT GTG TCT CAG TTT TGT C,对5株gtrI基因阳性的血清Y型菌株进行扩增。扩增产物包括完整的gtrI基因簇。PCR扩增产物经胶回收纯化后,送上海生物工程公司测序,并与已经公布的gtrI基因簇基因序列(GenBank accession no. JX509734)进行比对。
1.5 脉冲场凝胶电泳(pulsed field gel electrophoresis,PFGE)福氏志贺菌基因组PFGE分析按照参考文献[14]进行。基因组DNA用NotⅠ进行酶切,电泳在1% SKG上进行,电压6.0 V/cm,脉冲夹角120°,起始脉冲时间3 s,终止脉冲时间30 s,电泳时间18 h。完成电泳后,胶放入1 μg/ml溴化乙锭染色30 min,置纯水中脱色30 min,凝胶成像系统中成像。PFGE图像应用BioNumerics 4.0 数据库软件(AppliedMaths BVBA,Belium)进行处理和识别图像条带,聚类图类型运用非加权配对数学平均法(UPGMA)构建,采用Dice系数衡量PFGE带型之间的相似度。
2 结果对19972010年全国细菌性痢疾监测收集的2000余株福氏志贺菌进行血清型多重PCR检测,并与血清型玻片凝集结果进行比对。结果发现,有5株血清型Y菌株除了福氏志贺菌特征性wzx基因(782 bp)扩增阳性外,gtrI基因(1122 bp)扩增也阳性(图 1),但是I型抗血清凝集阴性;这5株菌除了与3(4)群抗血清凝集外,不与任何其他抗血清凝集,表现为血清Y型的特征(表 1)。其中3株(2002039、06HN364、10HN238)分别于2002、2006和2010年分离自河南;2株(06AH102、06AH120)于2006年分离自安徽。
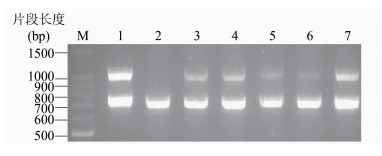
|
| 图 1 携带gtrI基因簇5株血清Y型福氏志贺菌多重PCR扩增电泳结果 Figure.1 Multiplex-PCR detection of 5 S. flexneriserotype Y strains carrying gtrIgene 注:1. 阳性对照2001019 (1a); 2. 阴性对照2003036(Y); 3. 06AH102(Y); 4. 06AH120(Y); 5. 2002039(Y); 6. 06HN364(Y); 7. 10HN238(Y)。gtrI基因扩增长度为1122 bp,wzx基因扩增长度为782 bp。 |
| 菌株
编号 |
血清型 | 血清
凝集谱 |
gtrI基因
PCR扩增结果 |
基因突变 |
| 06AH102 | Y | 3(4)+ | + | 1500 (T) 缺失 |
| 06AH120 | Y | 3(4)+ | + | 1175 (A) 缺失 |
| 2002039 | Y | 3(4)+ | + | 1512 (T) 缺失 |
| 06HN364 | Y | 3(4)+ | + | 1500 (T) 缺失 |
| 10HN238 | Y | 3(4)+ | + | 1175 (A) 缺失 |
采用引物gtrIAB对这5株菌株的gtrI基因簇进行扩增,结果5株菌株均得到全长2947 bp的扩增产物。扩增产物纯化后进行测序,测序结果与标准菌株的gtrI基因簇序列进行比对。结果发现,5株菌基因组中均含有gtrI基因簇序列,但是其中的gtrI基因存在功能失活性突变。菌株06HN364和06AH102的gtrI基因的1500位T碱基缺失,在编码蛋白序列500位产生终止密码子;菌株10HN238和06AH120的gtrI基因1175位A碱基缺失,在编码蛋白序列393位产生终止密码子;菌株2002039 gtrI基因的1512位T碱基缺失,在编码蛋白序列503位产生终止密码子(表 1)。与参考序列相比,gtrA和gtrB基因序列均未发现功能失活性突变。
对5株菌株进行PFGE分析,并与PFGE库中其他血清型福氏志贺菌进行聚类分析。结果发现,根据PFGE带谱,5株菌株可以分为5种不同的带型,但是都和血清1a型菌株具有相同或相近的带谱(图 2)。其中06AH102与1a血清型菌株05AH422、2005025、 2001038、06HN136具有相同的带谱;菌株10HN238与1a血清型菌株07HN162、08HN074、10HN128具有相同的带谱;菌株2002039与1a血清型菌株2002002和2002038具有完全相同的带谱。虽然没有发现与06HN364和06AH120带谱完全一致的菌株,但是他们与血清1a型菌株带谱相近,只有1条带的差异。这些结果提示这5株菌与1a血清型菌株的亲缘关系。
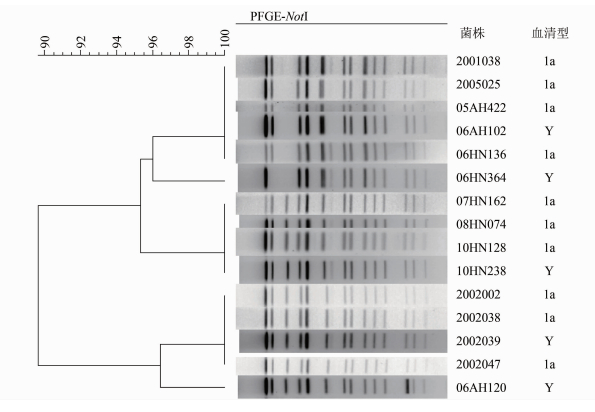
|
| 图 2 5株携带gtrI基因簇的Y血清型福氏志贺菌的PFGE带谱与1a血清型菌株的聚类关系 Figure.2 Relationship in clustering of PFGE patterns between 5 S. flexneri serotype Y strains carrying gtrIgene and serotype 1a strains |
通过携带O抗修饰基因或基因簇的血清型转换噬菌体(SfI、SfII、Sf6、SfIV、SfV、SfX、SfIC)的溶原性转化[11, 15, 16, 17, 18, 19, 20],或携带的磷酸乙醇胺转移酶编码基因opt的质粒(pSFxv-1)[4],或携带乙酰基转移酶编码基因oacB、oacC的前噬菌体样结构[21, 22],介导在四糖骨架不同糖基上通过不同方式链接乙酰基、糖基或磷酸乙醇胺基团,在细菌表面形成抗原表位Ⅰ、Ⅱ、Ⅲ、Ⅳ、Ⅴ、3;4、6、7;8、9、10、1C 和MASF Ⅳ-1[7]。不同抗原表位的组合形成了福氏志贺菌多样的血清型,这是福氏志贺菌血清型产生的分子机制。
本研究发现,5株福氏志贺菌Y血清型菌株中含有gtrI基因簇(gtrA,gtrB,gtrI),但是其中的gtrI基因发生功能性失活突变,这造成I型抗原表位的缺失和I型抗血清的凝集阴性,表现为Y血清型的特征;PFGE分析发现这5株菌与本实验室保存的福氏志贺菌血清型1a菌株具有相同或相似的PFGE带谱,提示这些菌株原来可能是血清1a型,在进化过程中通过gtrI基因的突变回复到Y血清型。
既往研究发现,福氏志贺菌O抗修饰基因突变导致的血清型回复突变也存在其他血清型中。Chen 等[23]在一株Y血清型菌株中发现gtrII基因簇,但是其中的gtrII基因发生失活突变;Roberts等[24]发现血清型Y菌株SFL124基因组中携带SfII前噬菌体基因组,但是其中的gtrII基因存在插入突变,导致功能失活;本研究也发现,在部分血清型Y和X菌株中,携带功能失活的gtrII基因;提示他们来源于2a、2b血清型菌株[9, 13]。另外,在一株5a血清型菌株中,笔者也发现携带功能失活的oac基因[13],导致群抗血清6凝集阴性,提示这株菌与3b血清型的渊源。此外,O抗修饰基因功能失活突变也在opt、oacB和oacD基因中发现,导致MASF Ⅳ-1 9和10抗原表位的缺失[21, 25]。这些结果提示福氏志贺菌O抗修饰基因突变失活造成的血清型转换是一种比较常见的现象,应该引起重视。
| [1] | Bardhan P, Faruque AS, Naheed A, et al. Decrease in shigellosis-related deaths without Shigella spp.-specific interventions, Asia[J].Emerg Infect Dis,2010,16(11):1718-1723. |
| [2] | Wang JP, Lan RT, Knirel YA, et al. Serological identification and prevalence of a novel O-antigen epitope linked to 3-and 4-O-acetylated rhamnose Ⅲ of lipopolysaccharide in Shigella flexneri[J]. J Clin Microbiol,2014,52(6):2033-2038. |
| [3] | Simmons DAR, Romanowska E. Structure and biology of Shigella flexneri O antigens[J]. J Med Microbiol,1987,23(4):289-302. |
| [4] | Sun QZ, Knirel YA, Lan RT, et al. A novel plasmid-encoded serotype conversion mechanism through addition of phosphoethanolamine to the O-antigen of Shigella flexneri[J]. PLoS One,2012,7(9):e46095. |
| [5] | Knirel YA, Lan RT, Senchenkova SN, et al. O-antigen structure of Shigella flexneri serotype Yv and effect of the lpt-O gene variation on phosphoethanolamine modification of S. flexneri O-antigens[J]. Glycobiology,2013,23(4):475-485. |
| [6] | Perepelov AV, Shekht ME, Liu B, et al. Shigella flexneri O-antigens revisited:final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity[J]. FEMS Immunol Med Microbiol,2012,66(2):201-210. |
| [7] | Sun QZ, Knirel YA, Lan RT, et al. Dissemination and serotype modification potential of pSFxv_2, an O-antigen PEtN modification plasmid in Shigella flexneri[J]. Glycobiology,2014,24(3):305-313. |
| [8] | Clark CA, Beltrame J, Manning PA. The oac gene encoding a lipopolysaccharide O-antigen acetylase maps adjacent to the integrase-encoding gene on the genome of Shigella flexneri bacteriophage Sf6[J]. Gene,1991,107(1):43-52. |
| [9] | Sun QZ, Lan RT, Wang JP, et al. Identification and characterization of a novel Shigella flexneri serotype Yv in China[J]. PLoS One,2013,8(7):e70238. |
| [10] | Allison GE, Verma NK. Serotype-converting bacteriophages and O-antigen modification in Shigella flexneri[J]. Trends Microbiol,2000,8(1):17-23. |
| [11] | Sun QZ, Lan RT, Wang YT, et al. Isolation and genomic characterization of SfI, a serotype-converting bacteriophage of Shigella flexneri[J]. BMC Microbiol,2013,13(1):39. |
| [12] | Wang JP, Luo X, Xu JG, et al. Preparation of antisera against PEtN modification on O-antigen of Shigella flexneri[J]. Disease Surveillance,2013,28(11):940-942.(in Chinese) 王建平,罗霞,徐建国,等.福氏志贺菌O-抗原磷酸乙醇胺修饰特异抗血清的制备[J]. 疾病监测,2013,28(11):940-942. |
| [13] | Sun QZ, Lan RT, Wang YT, et al. Development of a multiplex PCR assay targeting O-antigen modification genes for molecular serotyping of Shigella flexneri[J]. J Clin Microbiol,2011,49(11):3766-3770. |
| [14] | Ye CY, Lan RT, Xia SL, et al. Emergence of a new multidrug-resistant serotype X variant in an epidemic clone of Shigella flexneri[J]. J Clin Microbiol,2010,48(2):419-426. |
| [15] | Stagg RM, Tang SS, Carlin NIA, et al. A novel glucosyltransferase involved in O-antigen modification of Shigella flexneri serotype 1c[J]. J Bacteriol,2009,191(21):6612-6617. |
| [16] | George DT, Stephenson DP, Tran E, et al. Complete genome sequence of SfII, a serotype-converting bacteriophage of the highly prevalent Shigella flexneri serotype 2a[J]. Genome Announc,2013,1(5):e00626-13. |
| [17] | Allison GE, Angeles D, Tran-Dinh N, et al. Complete genomic sequence of SfV, a serotype-converting temperate bacteriophage of Shigella flexneri[J]. J Bacteriol,2002,184(7):1974-1987. |
| [18] | Jakhetia R, Talukder KA, Verma NK. Isolation, characterization and comparative genomics of bacteriophage SfIV:a novel serotype converting phage from Shigella flexneri[J]. BMC Genomics,2013,14:677. |
| [19] | Guan S, Bastin DA, Verma NK. Functional analysis of the O antigen glucosylation gene cluster of Shigella flexneri bacteriophage SfX[J]. Microbiology,1999,145(5):1263-1273. |
| [20] | Casjens S, Winn-Stapley DA, Gilcrease EB, et al. The chromosome of Shigella flexneri bacteriophage Sf6:complete nucleotide sequence, genetic mosaicism, and DNA packaging[J]. J Mol Biol,2004,339(2):379-394. |
| [21] | Wang JP, Knirel YA, Lan RT, et al. Identification of an O-acyltransferase gene(oacB) that mediates 3-and 4-O-acetylation of rhamnose Ⅲ in Shigella flexneri O antigens[J]. J Bacteriol,2014,196(8):1525-1531. |
| [22] | Knirel YA, Wang JP, Luo X, et al. Genetic and structural identification of an O-acyltransferase gene(oacC) responsible for the 3/4-O-acetylation on rhamnose Ⅲ in Shigella flexneri serotype 6[J]. BMC Microbiol,2014,14:266. |
| [23] | Chen JH, Hsu WB, Chiou CS, et al. Conversion of Shigella flexneri serotype 2a to serotype Y in a shigellosis patient due to a single amino acid substitution in the protein product of the bacterial glucosyltransferase gtrII gene[J]. FEMS Microbiol Letters,2003,224(2):277-283. |
| [24] | Roberts F, Jennison AV, Verma NK. The Shigella flexneri serotype Y vaccine candidate SFL124 originated from a serotype 2a background[J]. FEMS Immunol Med Microbiol,2005,45(2):285-289. |
| [25] | Sun QZ, Knirel YA, Wang JP, et al. Serotype-converting bacteriophage SfII encodes an acyltransferase protein that mediates 6-O-acetylation of GlcNAc in Shigella flexneri O-antigens, conferring on the host a novel O-antigen epitope[J]. J Bacteriol,2014,196(20):3656-3666. |
 2015, Vol. 30
2015, Vol. 30


