扩展功能
文章信息
- 汪永禄, 陶勇, 王利, 金文杰, 叶长芸, 刘丽云
- WANG Yong-lu, TAO Yong, WANG Li, JIN Wen-jie, YE Chang-yun, LIU Li-yun
- 13株杨氏枸橼酸杆菌的黏附、细胞毒性和耐药性检测
- Adhesion, cytotoxicity and antimicrobial resistance of 13 Citrobacter youngae isolates
- 疾病监测, 2017, 32(12): 953-957
- Disease Surveillance, 2017, 32(12): 953-957
- 10.3784/j.issn.1003-9961.2017.12.014
-
文章历史
- 收稿日期:2017-08-18
2. 北京科技大学化学与生物工程学院, 北京 100083;
3. 中国疾病预防控制中心传染病预防控制所传染病预防控制国家重点实验室, 北京 102206
2. School of Chemistry and Biological Engineering, University of Science and Technology Beijing, Beijing 100083, China;
3. State Key Laboratory for Communicable Disease Prevention and Control, Institute for Communicable Disease Prevention and Control, Chinese Center for Disease Control and Prevention, Beijing 102206, China
枸橼酸杆菌(Citrobacter)是革兰阴性杆菌,通常寄居在土壤、水、食物、人类和动物肠道中[1-2]。枸橼酸杆菌由于污染食物而引起散在感染和暴发[3-7]。弗氏枸橼酸杆菌的一些分离株通过获得毒力因子而引起人的腹泻或食物中毒[8]。既往的研究中,通过黏附和LDH实验,检测弗氏枸橼酸杆菌对HEp-2细胞的黏附和细胞毒性,发现1株强的聚集性黏附和细胞毒性的弗氏枸橼酸杆菌,通过测序发现该菌具有一个完整的Ⅵ型分泌系统[8]。杨氏枸橼酸杆菌(Citrobacter youngae,CY)作为枸橼酸杆菌属的一个种,很少引起感染。Chen等[9]报道CY可以引起腹膜炎。目前还没有报道CY引起腹泻的病例。
枸橼酸杆菌频繁引起医院内感染,耐药问题日益突出[10]。已经有文献报道分离到携带广谱β-内酰胺酶(ESBLs)和喹诺酮抗性基因的枸橼酸杆菌属[11-16]。
本研究对安徽省马鞍山市的腹泻病例分离到的13株CY进行黏附实验、细胞毒实验和药敏实验。以筛查出具有细胞毒性的CY,评估其致病性,同时评价13株CY的耐药情况。
1 材料与方法 1.1 材料 1.1.1 菌株和细胞来源2007年7-8月对安徽省马鞍山市的病原菌调查期间,从医院门诊腹泻病例粪便样本分离到13株CY。使用API 20E试纸条(法国bioMérieux公司)进行枸橼酸杆菌种的鉴定。所有分离株储存在甘油肉汤中,保存于-80 ℃。参考菌株CF74和CF72为本实验室保存。HEp-2细胞(人喉癌细胞系)购自ATCC细胞库。
1.1.2 主要试剂和仪器实验所用Gimesa染液购自sigma公司,Cyto-Tox 96 Cytoxicity Kit购自美国Promega公司;RPMI1640培养液和小牛血清购自GIBCO公司。药敏纸片购自OXOID公司。Steri-Cycle CO2培养箱购自Thermo Forma公司;BX51正置显微镜购自奥林巴斯公司。
1.2 方法 1.2.1 培养基及培养条件CY的常规培养条件:LB液体培养基(1%胰蛋白胨、0.5%酵母提取物、1%NaCl,调节pH值至7.4)及LBA固体平板(LB液体培养基中加入1.5%琼脂),37 ℃培养。HEp-2细胞的培养条件:含10%小牛血清的1640细胞培养液,37 ℃,5% CO2的温箱孵育。
1.2.2 CY黏附和细胞毒实验按照Bai等[8]对枸橼酸杆菌黏附HEp-2细胞的实验流程进行黏附实验,具体实验:HEp-2细胞接种于24孔细胞板,培养液为含10%FBS的1640,培养过夜。细菌感染细胞的感染复数(MOI)为100 : 1,在37 ℃,5%CO2的温箱中共同孵育3 h后,经过PBS清洗24孔板,每孔加入1% Gimesa染液作用30 min,纯水清洗24孔板3次,封片,显微镜观察。每个样品有3个平行实验,并且进行至少2次重复实验。黏附指数(<1;>1和<50;>50)指的是在显微镜下的10个视野下计数平均每个HEp-2细胞黏附的细菌数。
乳酸脱氢酶(lactate dehydrogenase,LDH)细胞毒性检测实验方法按照Cytotox 96 kit (Promega)操作说明进行。具体步骤:HEp-2细胞接种于96孔板,在含10%的FFBS的1640培养基中培养过夜;次日,细菌感染细胞的MOI为100 : 1,在37 ℃,5% CO2的温箱中共同孵育8 h。离心,移取每孔上清50 μl至另一96孔板,并在每孔加入50 μl LDH反应液,避光反应30 min(室温15~25 ℃);每孔加入50 μl LDH终止液,酶标仪测吸光度(A),测量波长为490 nm,参考波长为630 nm。低对照组为不加细菌的细胞培养液,高对照组为细胞被加入裂解液完全裂解(promege试剂盒带,在显色前40 min加入1×lysis)。
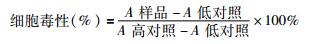
|
每个样品有3个平行实验,并且进行至少2次重复实验。
1.2.3 药敏试验根据美国临床实验室标准委员会(CLSI)推荐的改良K-B纸片法进行。15种药敏纸片分别是氨苄西林(10 μg)、氨苄西林/舒巴坦(20 μg)、氨曲南(30 μg)、头孢唑啉(30 μg)、头孢吡肟(30 μg)、头孢他啶(30 μg)、头孢曲松(30 μg)、环丙沙星(5 μg)、庆大霉素(10 μg)、亚胺培南(10 μg)、左旋氧氟沙星(5 μg)、呋喃妥因(300 μg)、哌拉西林/他唑巴坦(100 μg/10 μg)、妥布霉素(10 μg)和复方新诺明(25 μg)。药敏试验用M-H培养基及药敏纸片法均由英国OXOID公司生产。药敏质控菌株大肠埃希菌ATCC25922由本实验室保存,药敏结果判定参照2016年发布的CLSI标准。
1.2.4 统计学分析采用SPSS 13.0软件包(SPSS Inc.,Chicago,IL,USA)进行数据统计分析。非参数检验(Mann-Whitney U-test)用于组间的差异比较分析,P<0.05为差异有统计学意义。
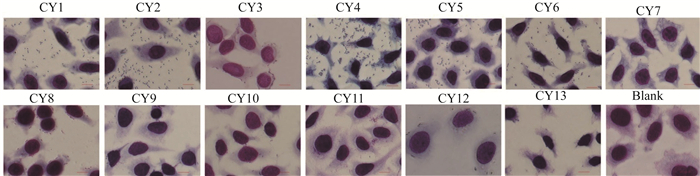
2 结果 2.1 CY对HEp-2细胞的黏附作用通过比较13株CY对HEp-2细胞的黏附差异,见图 1。5株CY(CY7、CY9、CY10、CY11和CY13)几乎没有黏附性,黏附指数<1。6株CY(CY1、CY2、CY3、CY6、CY8和CY12)具有散在的黏附性,黏附指数>1,但<50。2株CY(CY4和CY5)具有强的黏附性,黏附指数>50。
|
| 图 1 杨氏枸橼酸杆菌粘附HEp-2细胞 Figure 1 Adhesion of C. youngae isolates on HEp-2 cell 注:光学显微镜下观察13株CY黏附HEp-2细胞的情况Blank:不加菌的细胞作为黏附的空白对照,Bar: 10 mm(革兰染色,×1 000) |
| |
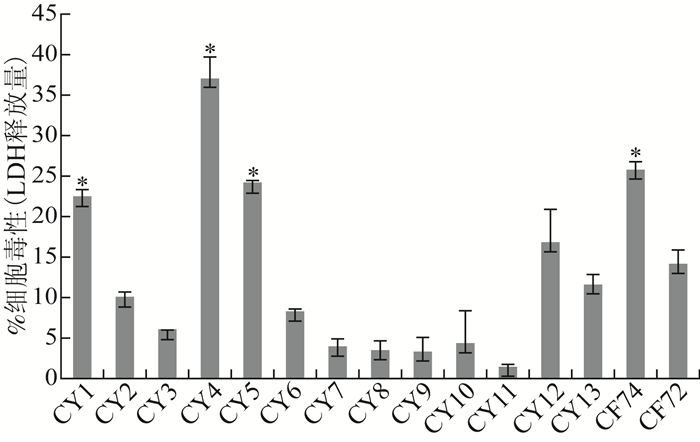
通过比较13株CY对HEp-2细胞的细胞毒作用,在与HEp-2细胞作用8 h后(图 2和表 1),CY1引起的LDH释放量为22.37%,CY4为37.08%,CY5为24.05%,阳性对照CF74为25.65%,均明显高于阴性对照CF72引起的LDH释放量14.21%(均P=0.001)。其他10株CY引起的LDH释放量均<20.00%。
|
| 图 2 杨氏枸橼酸杆菌对HEp-2细胞的细胞毒作用 Figure 2 Cytotoxicity of C. youngae to HEp-2 cell 注:HEp-2细胞暴露于13株CY 8 h后,采用非参数检验(Mann-Whitney U-test)比较分析CF74与13株CY引起LDH释放量 |
| |
| 菌株名称 | LDH (%±平均误差值) |
| CY1 | 22.37±1.08 |
| CY2 | 9.72±0.79 |
| CY3 | 5.89±0.05 |
| CY4 | 37.08+2.55 |
| CY5 | 24.05±0.42 |
| CY6 | 8.15±0.49 |
| CY7 | 3.72±1.19 |
| CY8 | 3.40±1.29 |
| CY9 | 3.16±1.85 |
| CY10 | 4.19±4.22 |
| CY11 | 1.30±0.50 |
| CY12 | 16.51±4.13 |
| CY13 | 11.54±1.28 |
| CF74 | 25.65±1.20 |
| CF72 | 14.21±2.01 |
| 注:LDH (%±平均误差值):HEp-2细胞的乳酸脱氢酶释放情况 | |
用15种抗生素对13株CY进行药敏检测(表 2),13株CY对环丙沙星和妥布霉素均敏感(100%),其次是头孢他啶、头孢曲松、庆大霉素、亚胺培南和左旋氧氟沙星(92.30%)。13株CY对氨苄西林和头孢唑啉的耐药率最高(92.30%),其次是氨苄西林/舒巴坦(46.20%)、复方新诺明(38.50%)和氨曲南(30.80%)。CY1耐药率最高,为53.80%。
| 菌株名称 | 氨苄西林 | 氨苄西林/舒巴坦 | 氨曲南 | 头孢唑啉 | 头孢吡肟 | 头孢他啶 | 头孢曲松 | 环丙沙星 | 庆大霉素 | 亚胺培南 | 左旋氧氟沙星 | 呋喃妥因 | 哌拉西林/他唑巴坦 | 妥布霉素 | 复方新诺明 |
| CY1 | S | S | R | R | S | R | S | S | S | S | R | R | R | S | R |
| CY2 | R | S | S | R | S | S | S | S | S | R | S | S | S | S | R |
| CY3 | R | I | S | R | R | S | S | S | S | S | S | S | S | S | S |
| CY4 | R | R | S | R | S | S | S | S | S | S | S | S | S | S | S |
| CY5 | R | R | S | R | R | S | S | S | S | S | S | S | S | S | S |
| CY6 | R | I | R | R | S | S | S | S | S | S | S | S | S | I | R |
| CY7 | R | S | R | R | S | I | S | S | R | R | R | S | S | S | S |
| CY8 | R | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| CY9 | R | R | S | R | S | S | S | S | S | S | S | R | R | S | S |
| CY10 | R | R | S | R | S | S | S | S | S | S | S | S | S | S | S |
| CY11 | R | R | S | R | S | S | S | S | S | S | S | S | S | S | R |
| CY12 | R | R | R | R | S | S | S | S | S | S | S | S | S | S | R |
| CY13 | R | I | S | R | S | S | R | S | S | S | S | R | S | S | S |
| 注:R代表耐药,S代表敏感,I代表中介 | |||||||||||||||
枸橼酸杆菌作为条件致病菌,可引起包括尿道感染、肠道感染和脑膜炎等感染性疾病的发生[17-19]。弗氏枸橼酸杆菌在枸橼酸杆菌感染中最常见,通常与肠胃炎暴发和食源性疾病暴发相关。已有文献报道,弗氏枸橼酸杆菌可通过获得毒力基因而变成致病菌[8]。CY很少引起感染。目前还没有报道CY引起腹泻的病例。黏附是病原菌致病的关键步骤之一,黏附性的检测已经是体外检测法的一种[20]。我们从腹泻病例分离到13株CY,其中有8株CY具有中等以上黏附性,并且其中3株菌具有强的细胞毒性。
枸橼酸杆菌频繁引起医院内感染,而变成耐药菌,部分菌株呈现多重耐药的特点[10]。Leski等[21]报道在门诊病例中,从病例尿道分离到的弗氏枸橼酸杆菌大多数是多重耐药菌。Moges等[22]在医院和医院外的废水中分离到13株多重耐药的枸橼酸杆菌。枸橼酸杆菌的耐药主要是对β-内酰胺类抗生素和喹诺酮类抗生素的耐药[11-16]。在世界范围内,耐β-内酰胺类抗生素的枸橼酸杆菌在0.50%~36.00%之间[23-25]。在韩国、日本和美国,弗氏枸橼酸杆菌β-内酰胺酶携带率分别为4.90%~20.60%、0.20%~4.60%和0.90%,在美国和日本,科氏枸橼酸杆菌β-内酰胺酶携带率分别为3.50%和60.00%[11-13]。喹诺酮抗性基因主要包括qnr和aac(6′)-Ibcr基因[12, 15]。在中国,弗氏枸橼酸杆菌携带qnr和aac(6′)-Ibcr基因分别为72.80%和11.60%[15];在韩国,弗氏枸橼酸杆菌携带qnr基因为38.40%[12]。Park等[10]发现38.40%弗氏枸橼酸杆菌携带qnr基因。Yang等[26]发现弗氏枸橼酸杆菌对qnr和aac(6′)-Ib-cr基因的携带率分别是63.30%和26.70%。Zhang等[15]发现布氏枸橼酸杆菌对qnr和aac(6′)-Ib-cr基因的携带率是42.90%。我们分离的13株CY主要对一代头孢耐药,耐药率高达92.30%。有7株CY对3种以上抗生素耐药,是多重耐药菌株。其中2株具有强的细胞毒性。这些抗生素耐药性出现将对枸橼酸的感染治疗带来严重影响,提示需要重视并加强对枸橼酸杆菌耐药性的监测。
作者贡献:
汪永禄 ORCID:0000-0003-0937-6365
汪永禄:药敏实验
陶勇、王利:菌株分离鉴定
金文杰:细胞粘附和细胞毒性实验
叶长芸:实验指导
刘丽云:实验设计和文章撰写
| [1] |
Lipsky BA, Hook Ⅲ EW, Smith AA, et al. Citrobacter infections in humans:experience at the seattle veterans administration medical center and a review of the literature[J]. Rev Infect Dis, 1980, 2(5): 746-760. DOI:10.1093/clinids/2.5.746 |
| [2] |
Nada T, Baba H, Kawamura K, et al. A small outbreak of third generation cephem-resistant Citrobacter freundii infection on a surgical ward[J]. Jpn J Infect Dis, 2004, 57(4): 181-182. |
| [3] |
Guerrant RL, Dickens MD, Wenzel RP, et al. Toxigenic bacterial diarrhea:nursery outbreak involving multiple bacterial strains[J]. J Pediatr, 1976, 89(6): 885-891. DOI:10.1016/S0022-3476(76)80591-4 |
| [4] |
Tschäpe H, Prager R, Streckel W, et al. Verotoxinogenic Citrobacter freundii associated with severe gastroenteritis and cases of haemolytic uraemic syndrome in a nursery school:green butter as the infection source[J]. Epidemiol Infect, 1995, 114(3): 441-450. DOI:10.1017/S0950268800052158 |
| [5] |
Warner RD, Carr RW, McCleskey FK, et al. A large nontypical outbreak of norwalk virus. Gastroenteritis associated with exposing celery to nonpotable water and with Citrobacter freundii[J]. Arch Intern Med, 1991, 151(12): 2419-2424. DOI:10.1001/archinte.1991.00400120061010 |
| [6] |
Doulgeraki AI, Paramithiotis S, Nychas GJE. Characterization of the Enterobacteriaceae community that developed during storage of minced beef under aerobic or modified atmosphere packaging conditions[J]. Int J Food Microbiol, 2011, 145(1): 77-83. DOI:10.1016/j.ijfoodmicro.2010.11.030 |
| [7] |
Giammanco GM, Aleo A, Guida I, et al. Molecular epidemiological survey of Citrobacter freundii misidentified as Cronobacter spp. (Enterobacter sakazakii) and enterobacter hormaechei isolated from powdered infant milk formula[J]. Foodborne Pathog Dis, 2011, 8(4): 517-525. DOI:10.1089/fpd.2010.0719 |
| [8] |
Bai L, Xia SL, Lan RT, et al. Isolation and characterization of cytotoxic, aggregative Citrobacter freundii[J]. PLoS One, 2012, 7(3): e33054. DOI:10.1371/journal.pone.0033054 |
| [9] |
Chen KJ, Chen TH, Sue YM. Citrobacter youngae and Pantoea agglomerans peritonitis in a peritoneal dialysis patient[J]. Perit Dial Int, 2013, 33(3): 336-337. DOI:10.3747/pdi.2012.00151 |
| [10] |
Park YJ, Yu JK, Lee S, et al. Prevalence and diversity of qnr alleles in AmpC-producing Enterobacter cloacae, Enterobacter aerogenes, Citrobacter freundii and Serratia marcescens:a multicentre study from Korea[J]. J Antimicrob Chemother, 2007, 60(4): 868-871. DOI:10.1093/jac/dkm266 |
| [11] |
Park YJ, Park SY, Oh EJ, et al. Occurrence of extended-spectrum β-lactamases among chromosomal AmpC-producing Enterobacter cloacae, Citrobacter freundii, and Serratia marcescens in Korea and investigation of screening criteria[J]. Diagn Microbiol Infect Dis, 2005, 51(4): 265-269. DOI:10.1016/j.diagmicrobio.2004.11.009 |
| [12] |
Moland ES, Hanson ND, Black JA, et al. Prevalence of newer β-lactamases in gram-negative clinical isolates collected in the United States from 2001 to 2002[J]. J Clin Microbiol, 2006, 44(9): 3318-3324. DOI:10.1128/JCM.00756-06 |
| [13] |
Choi SH, Lee JE, Park SJ, et al. Prevalence, microbiology, and clinical characteristics of extended-spectrum β-lactamase-producing Enterobacter spp., Serratia marcescens, Citrobacter freundii, and Morganella morganii in Korea[J]. Eur J Clin Microbiol Infect Dis, 2007, 26(8): 557-561. DOI:10.1007/s10096-007-0308-2 |
| [14] |
Kanamori H, Yano H, Hirakata Y, et al. High prevalence of extended-spectrum β-lactamases and qnr determinants in Citrobacter species from Japan:dissemination of CTX-M-2[J]. J Antimicrob Chemother, 2011, 66(10): 2255-2262. DOI:10.1093/jac/dkr283 |
| [15] |
Zhang R, Ichijo T, Huang YL, et al. High prevalence of qnr and aac(6')-Ib-cr genes in both water-borne environmental bacteria and clinical isolates of Citrobacter freundii in China[J]. Microbes Environ, 2012, 27(2): 158-163. DOI:10.1264/jsme2.ME11308 |
| [16] |
Bae IK, Park I, Lee JJ, et al. Novel variants of the qnrB gene, qnrB22 and qnrB23, in Citrobacter werkmanii and Citrobacter freundii[J]. Antimicrob Agents Chemother, 2010, 54(7): 3068-3069. DOI:10.1128/AAC.01339-09 |
| [17] |
Gupta N, Yadav A, Choudhary U, et al. Citrobacter bacteremia in a tertiary care hospital[J]. Scand J Infect Dis, 2003, 35(10): 765-768. DOI:10.1080/00365540310016376 |
| [18] |
Samonis G, Karageorgopoulos DE, Kofteridis DP, et al. Citrobacter infections in a general hospital:characteristics and outcomes[J]. Eur J Clin Microbiol Infect Dis, 2000, 28(1): 61-68. |
| [19] |
Leski TA, Taitt CR, Bangura U, et al. Finished genome sequence of the highly multidrug-resistant human urine isolate Citrobacter freundii strain SL151[J]. Genome Announc, 2016, 4(6): e01225-16. DOI:10.1128/genomeA.01225-16 |
| [20] |
Mange JP, Stephan R, Borel N, et al. Adhesive properties of Enterobactersakazakii to human epithelial and brain microvascular endothelial cells[J]. BMC Microbiol, 2006, 6: 58. DOI:10.1186/1471-2180-6-58 |
| [21] |
Leski TA, Taitt CR, Bangura U, et al. High prevalence of multidrug resistant Enterobacteriaceae isolated from outpatient urine samples but not the hospital environment in Bo, Sierra Leone[J]. BMC Infect Dis, 2016, 16: 167. DOI:10.1186/s12879-016-1495-1 |
| [22] |
Moges F, Endris M, Belyhun Y, et al. Isolation and characterization of multiple drug resistance bacterial pathogens from waste water in hospital and non-hospital environments, Northwest Ethiopia[J]. BMC Res Notes, 2014, 7: 215. DOI:10.1186/1756-0500-7-215 |
| [23] |
Ali AM, Rafi S, Qureshi AH. Frequency of extended spectrum beta lactamase producing gram negative bacilli among clinical isolates at clinical laboratories of Army Medical College, Rawalpindi[J]. J Ayub Med Coll Abbottabad, 2004, 16(1): 35-37. |
| [24] |
Fernandes R, Amador P, Oliveira C, et al. Molecular characterization of ESBL-producing Enterobacteriaceae in northern Portugal[J]. Sci World J, 2014, 2014: 782897. |
| [25] |
Praharaj AK, Khajuria A, Kumar M, et al. Phenotypic detection and molecular characterization of beta-lactamase genes among Citrobacter species in a tertiary care hospital[J]. Avicenna J Med, 2016, 6(1): 17-27. DOI:10.4103/2231-0770.173578 |
| [26] |
Yang H, Chen HB, Yang QW, et al. High prevalence of plasmid-mediated quinolone resistance genes qnr and aac(6')-Ib-cr in clinical isolates of Enterobacteriaceae from nine teaching hospitals in China[J]. Antimicrob Agents Chemother, 2008, 52(12): 4268-4273. DOI:10.1128/AAC.00830-08 |
 2017, Vol. 32
2017, Vol. 32


