扩展功能
文章信息
- 陈美玲, 卢昕, 赵林, 李杰, 阚飙, 逄波
- Chen Meiling, Lu Xin, Zhao Lin, Li Jie, Kan Biao, Pang Bo
- 我国东南沿海地区副溶血弧菌O3:K6血清型耐药分析
- Antibiotic resistance of Vibrio parahaemolyticus serotype O3:K6 in southeastern coastal area of China
- 疾病监测, 2018, 33(5): 365-369
- Disease Surveillance, 2018, 33(5): 365-369
- 10.3784/j.issn.1003-9961.2018.05.005
-
文章历史
- 收稿日期:2018-02-01
本期特邀专题主持人——崔志刚副研究员:
副研究员,硕士,中国疾病预防控制中心传染病预防控制所国家致病菌识别网中心实验室副主任。主要从事传染病预防控制相关工作和研究,包括现场疫情处理、传染病监测信息管理、生物信息学研究尤其是传染病实验室监测信息化研究,负责研发的传染病实验室监测数据分析和传输系统获得12项软件著作权,申报发明专利2项。近年来,作为子课题负责人完成十一五和十二五科技重大专项各一项,作为主要负责人建立了覆盖全国疾控系统的实验室分子分型监测网络,参与筹划和建立了国家致病菌识别网。历年参与处置霍乱、SARS、猪链球菌感染、流脑暴发等重要传染病疫情防控工作,在PLos One、J Clin Microbiol、BMC Microbiology、中华流行病学杂志、疾病监测、中国预防医学和实用预防医学等国内外核心期刊以第一作者/通信作者发表有关研究论文10余篇, 参与编写专著2部。
副溶血弧菌(Vibrio parahaemolyticus)是一种嗜盐性革兰阴性菌,广泛存在于水体、水底沉积物和水生动物体内,其产生3类溶血素,即不耐热溶血素(thermolabile hemolysin,TLH)、耐热直接溶血素(thermostable direct hemolysin,TDH)和耐热相关溶血素(thermostable direct hemolysin-related hemolysin,TRH)。TDH和TRH是副溶血弧菌产生的重要致病因子[1],TDH具有溶血、肠毒性、细胞毒性;trh基因与尿素酶的产生密切相关。一般认为tdh和(或)trh基因呈阳性的菌株为致病株,两者均呈阴性的菌株为非致病菌株[2]。
副溶血弧菌感染在全球大多国家和地区广泛流行,也是亚洲部分国家和地区细菌食源性疾病的首要致病菌。自1998年以来,副溶血弧菌逐渐成为我国食源性疾病的主要致病菌[3]。该菌O3:K6血清型于1996年在印度首次被分离,后该血清型及其变异型在世界广泛传播[4]。
大部分副溶血弧菌感染病例为自限性,无需使用抗生素[5]。但因抗生素的不规范使用,细菌耐药问题日益严重,使大多数国家和地区分离的菌株有多药耐药(multidrug resist,MDR)现象,如美国[6]、马来西亚[7]和韩国[8]等。MDR指对不少于3类(每类≥ 1种)抗生素耐药[9]。MDR菌株的出现使常规抗生素失去疗效,为临床治疗带来巨大挑战。我国东南沿海地区为副溶血弧菌感染高发地区,本研究选取2002-2016年分离自该地的O3:K6型菌株,分析其毒力基因携带情况及耐药现状,现将结果报告如下。
1 材料与方法 1.1 菌株来源选取我国东南沿海地区2002- 2016年分离的O3:K6血清型副溶血弧菌,共77株(上海市42株、深圳市21株、江苏省9株、浙江省5株)。其中食品分离株5株、临床腹泻患者分离株72株。副溶血弧菌ATCC33847(tdh+,trh-)和ATCC17082(tdh-,trh+)标准菌株和大肠埃希菌(Escherichia coli)ATCC25992保存于中国疾病预防控制中心传染病预防控制所国家重点实验室。
1.2 毒力基因扩增水煮法获得核酸模板:挑取单菌落于100 μl灭菌纯水中,振荡混匀,煮沸10 min,冰上放置5 min,离心10 min,取上清液作为PCR反应模板。采用美国国立生物技术信息中心(NCBI)提供的Primer-Blast功能设计引物,引物序列及产物大小见表 1。反应体系:采用20 μl反应体系,其中含正、反向引物各1 μl(浓度为10 μmol/ml),Taq酶(TaKaRa)10 μl,DNA模板1 μl,去离子水补足20 μl。以水为阴性对照,以标准菌株ATCC33847为tdh阳性对照,以标准菌株ATCC17082为trh阳性对照。反应条件:95 ℃预变性5 min,95 ℃ 30 s、60 ℃ 60 s、72 ℃ 60 s,25个循环,72 ℃延伸10 min。取3 μl PCR产物进行1%琼脂糖凝胶电泳,观察是否扩增出条带。
| 毒力基因 | 引物名称 | 引物序列(5'~3') | 产物大小 (bp) |
| tdh | tdh-F | TGG CTG CAT TCA AAA CAT CTG C | 305 |
| tdh-R | CAC AGC AGA ATG ACC GCT CT | ||
| trh | trh-F | TTG GCT TCG ATA TTT TCA GTA TCT | 484 |
| trh-R | CAT AAC AAA CAT ATG CCC ATT TCC G |
采用世界卫生组织(WHO)推荐的K-B纸片法进行药敏试验,结合美国临床实验室标准化协会(CLSI)的抗生素选择原则和我国抗生素的使用情况,选择氨苄西林(AMP,10 μg)、头孢吡肟(FEP,10 μg)、头孢西丁(FOX,30 μg)、头孢曲松(CRO,30 μg)、亚胺培南(IPM,10 μg)、庆大霉素(GEN,10 μg)、卡那霉素(KAN,10 μg)、链霉素(STR,10 μg)、阿奇霉素(AZM,15 μg)、环丙沙星(CIP,5 μg)、萘啶酸(NAL,10 μg)、多西环素(DOX,10 μg)、氯霉素(CHL,30 μg)、磺胺甲恶唑(SOX,10 μg)、复方新诺明(SXT,10 μg)共15种药敏纸片(OXOID)进行药敏试验。结果判读参照CLSI M45弧菌属(Vibrio spp.)[10]和CLSI M100S肠杆菌科(Enterobacteriaceae)[11]2016版标准,以ATCC25922为质控菌株,将菌株对每种抗生素的耐药情况定为敏感、中介和耐药。
2 结果 2.1 毒力基因携带情况77株O3:K6型副溶血弧菌中,tdh基因阳性菌株73株,阳性率为94.81% (73 / 77),其中患者分离株tdh基因携带率为97.22%(70/72)。仅有1株为trh阳性,阳性率为1.30%(1/77),部分样品PCR结果见图 1。致病株73株,包括72株tdh+ trh-菌株和1株tdh+ trh+菌株,见表 2。
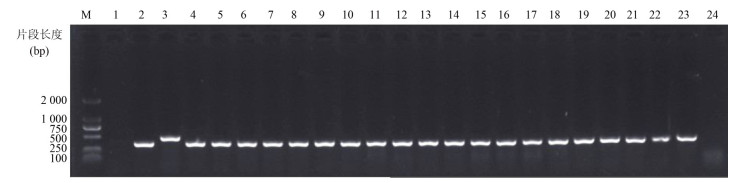
|
| 图 1 部分副溶血弧菌tdh和trh基因PCR结果 Figure 1 Electrophoresis map of PCR products(tdh and trh)from some V. Parahaemolyticus strains 注:M.DL2000 DNA Marker;1.阴性对照;2.ATCC33847(tdh阳性对照);3.ATCC17082(trh阳性对照);4~24.实验菌株 |
| |
| 分类 | tdh | trh | 临床菌株数 | 环境菌株数 | 合计 |
| 致病株 | + | - | 69 | 3 | 72 |
| - | + | 0 | 0 | 0 | |
| + | + | 1 | 0 | 1 | |
| 非致病株 | - | - | 2 | 2 | 4 |
77株O3:K6型副溶血弧菌对AMP、STR、KAN、SOX、CIP的敏感率较低,分别为7.79%、18.18%、31.17%、49.35%、54.55%,其中对AMP、SOX和STR的耐药率最高,分别为84.42%、36.36%、32.47%;对CRO、FOX、FEP、GEN、AZM的敏感率较高,分别为96.10%、87.01%、81.82%、97.40%、93.51%;所有菌株对IPM、SXT、NAL、DOX、CHL均敏感,见表 3。
| 药品名称 | 非敏感 | 敏感 | ||||||||
| 耐药 | 中介 | 合计 | ||||||||
| 数量(株) | 耐药率(%) | 数量(株) | 耐药率(%) | 数量(株) | 耐药率(%) | 数量(株) | 耐药率(%) | |||
| 氨苄西林 | 65 | 84.42 | 6 | 7.79 | 71 | 92.21 | 6 | 7.79 | ||
| 头孢西丁 | 0 | 0.00 | 10 | 12.99 | 10 | 12.99 | 67 | 87.01 | ||
| 头孢曲松 | 1 | 1.30 | 2 | 2.60 | 3 | 3.90 | 74 | 96.10 | ||
| 头孢吡肟 | 1 | 1.30 | 13 | 16.88 | 14 | 18.18 | 63 | 81.82 | ||
| 亚胺培南 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 77 | 100.00 | ||
| 阿奇霉素 | 5 | 6.49 | 0 | 0.00 | 5 | 6.49 | 72 | 93.51 | ||
| 链霉素 | 25 | 32.47 | 38 | 49.35 | 63 | 81.82 | 14 | 18.18 | ||
| 庆大霉素 | 0 | 0.00 | 2 | 2.60 | 2 | 2.60 | 75 | 97.40 | ||
| 卡那霉素 | 0 | 0.00 | 53 | 68.83 | 53 | 68.83 | 24 | 31.17 | ||
| 环丙沙星 | 1 | 1.30 | 34 | 44.16 | 35 | 45.45 | 42 | 54.55 | ||
| 萘啶酸 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 77 | 100.00 | ||
| 多西环素 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 77 | 100.00 | ||
| 氯霉素 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 77 | 100.00 | ||
| 磺胺异恶唑 | 28 | 36.36 | 11 | 14.29 | 39 | 50.65 | 38 | 49.35 | ||
| 复方新诺明 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 77 | 100.00 | ||
11株菌株的MDR率为14.29%(11/77),均为致病株,包括10株患者分离株和1株食品分离株。其中7株菌对3类抗生素耐药,耐药谱为AMP-STR- SOX,3株耐药谱为AMP-AZM-SOX,1株2007年深圳市患者分离株对5种4类抗生素耐药,耐药谱为AMP-CRO-FEP-STR-CIP。
3 讨论TDH和TRH是副溶血弧菌产生的重要致病因子,分离自腹泻患者的副溶血弧菌大多可产生TDH和(或)TRH,而环境或食品分离株多不具有该特性[12]。本研究O3:K6型副溶血弧菌的tdh基因携带率为94.81%,其中临床腹泻患者分离株的tdh基因携带率为97.22%,食品分离株tdh基因携带率为60.00%,trh基因携带率为1.30%。宋晶玲[13]研究发现,浙江省189株O3:K6型副溶血弧菌的tdh基因携带率为99.45%(182/183),trh基因携带率为0.55% (1/183);墨西哥西北海岸分离的O3:K6型副溶血弧菌中,65.20%(30 / 46)环境菌株携带tdh基因,97.2%(241/249)临床菌株携带tdh基因[14],均与本研究结果相似。
目前,O3:K6血清型副溶血弧菌在世界广泛传播流行,是我国报道的副溶血弧菌患者分离株最常见的血清型。O3:K6型菌对AMP的耐药率较高,对头孢唑林、头孢呋辛等头孢菌素中度耐药,对其他大部分常用抗生素(如美罗培南、左氧氟沙星等)比较敏感[15]。
本研究发现,副溶血弧菌对AMP耐药最严重,与世界各国情况比较相似。美国早在1978年发现其对AMP有较高的耐药率[12]。目前,全球大多国家分离的副溶血弧菌普遍对青霉素类抗生素有较高的耐药率。同时发现,81.82%菌株对水产养殖中常用的氨基糖苷类抗生素STR不敏感,其中耐药率达32.47%;69.83%菌株对KAN不敏感。中国[16]和韩国[17]水产品及印度[18]患者分离的副溶血弧菌均对STR有较高的耐药率。此外,在许多发展中国家水产品中也发现其对KAN产生较高耐药率[19-22]。45.45%菌株对于常用于临床治疗腹泻的喹诺酮类抗生素CIP不敏感,而意大利腹泻患者分离株对CIP的耐药率高达62.50%[23]。
77株O3:K6型副溶血MDR弧菌中有11株为MDR菌株(MDR率达14.29%),且全部为致病株;患者分离株MDR率为13.89%,远高于李薇薇等[24]于2007-2009年自四川、江苏、辽宁、浙江、广西地区腹泻患者分离的副溶血弧菌MDR率(0.00%),但低于Chen等[25]于2006-2013年我国东南沿海急性腹泻患者分离株的多药耐药率(28.00%)。研究发现1株患者分离株对5种抗生素耐药,其耐药谱为AMP-CRO-FEP-STR-CIP,其耐药机制有待进一步研究。
综上所述,我国东南沿海地区的77株O3:K6型副溶血弧菌存在MDR现象,MDR菌株全部为致病菌株。受试菌株对AMP的耐药率最高。对氨基糖苷类抗生素中的STR和KAN以及临床治疗腹泻常用的喹诺酮类抗生素CIP的敏感率较低。对以FOX、CRO、FEP为代表的二代、三代、四代头孢菌素、碳青霉烯类(IPM)、大环内酯类(AZM)、四环素类(DOX)和苯丙醇类(CHL)等临床常用抗生素比较敏感。
作者贡献:
陈美玲 ORCID:0000-0002-7244-1724
陈美玲:实验操作,数据分析,论文写作
卢昕:实验指导,论文指导
赵林:实验操作
李杰:实验指导
阚飙:课题指导
逄波:课题设计,实验指导,论文指导
| [1] |
Raghunath P. Roles of thermostable direct hemolysin(TDH) and TDH-related hemolysin(TRH) in Vibrio parahaemolyticus[J]. Front Microbiol, 2014, 5: 805. DOI:10.3389/fmicb.2014.00805 |
| [2] |
He PY, Chen ZW, Luo JY, et al. Multiplex real-time PCR assay for detection of pathogenic Vibrio parahaemolyticus strains[J]. Mol Cell Probes, 2014, 28(5/6): 246-250. DOI:10.1016/j.mcp.2014.06.001 |
| [3] |
Wu YN, Wen J, Ma Y, et al. Epidemiology of foodborne disease outbreaks caused by Vibrio parahaemolyticus, China, 2003-2008[J]. Food Control, 2014, 46: 197-202. DOI:10.1016/j.foodcont.2014.05.023 |
| [4] |
Nair GB, Ramamurthy T, Bhattacharya SK, et al. Global dissemination of Vibrio parahaemolyticus serotype O3:K6 and its serovariants[J]. Clin Microbiol Rev, 2007, 20(1): 39-48. DOI:10.1128/CMR.00025-06 |
| [5] |
Yan WX, Dai Y, Zhou YJ, et al. Risk factors for sporadic Vibrio parahaemolyticus gastroenteritis in east China:a matched case-control study[J]. Epidemiol Infect, 2015, 143(5): 1020-1028. DOI:10.1017/S0950268814001599 |
| [6] |
Urquhart EA, Jones SH, Yu JW, et al. Environmental conditions associated with elevated Vibrio parahaemolyticus concentrations in Great Bay Estuary, New Hampshire[J]. PLoS One, 2016, 11(5): e0155018. DOI:10.1371/journal.pone.0155018 |
| [7] |
Tan CW, Malcolm TTH, Kuan CH, et al. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from short mackerels(Rastrelliger brachysoma) in Malaysia[J]. Front Microbiol, 2017, 8: 1087. DOI:10.3389/fmicb.2017.01087 |
| [8] |
Jang HM, Kim YB, Choi S, et al. Prevalence of antibiotic resistance genes from effluent of coastal aquaculture, South Korea[J]. Environ Pollut, 2017, 233: 1049-1057. DOI:10.1016/j.envpol.2017.10.006 |
| [9] |
Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria:an international expert proposal for interim standard definitions for acquired resistance[J]. Clin Microbiol Infect, 2012, 18(3): 268-281. DOI:10.1111/j.1469-0691.2011.03570.x |
| [10] |
Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious Bacteria. 3rd ed. CLSI guideline M45[S]. Clinical and Laboratory Standards Institute, 2016.
|
| [11] |
Clinical and Laboratory Standards Institute. CLSI M100-S26 performance standards for antimicrobial susceptibility testing: twenty-sixth edition[S]. Clinical and Laboratory Standards Institute, 2016.
|
| [12] |
Johnson CN, Flowers AR, Young VC, et al. Genetic relatedness among tdh+ and trh+ Vibrio parahaemolyticus cultured from Gulf of Mexico oysters(Crassostrea virginica) and surrounding water and sediment[J]. Microb Ecol, 2009, 57(3): 437-443. DOI:10.1007/s00248-008-9418-3 |
| [13] |
宋晶玲. 240株O3血清型副溶血弧菌tdh和trh毒力基因研究[J]. 中国食品卫生杂志, 2017, 29(4): 423-427. Song JL. The pathogenicity of 240 Vibrio parahaemolyticus serovar O3 strains[J]. Chin J Food Hygiene, 2017, 29(4): 423-427. DOI:10.13590/j.cjfh.2017.04.007 |
| [14] |
de Jesús Hernández-Díaz L, Leon-Sicairos N, Velazquez-Roman J, et al. A pandemic Vibrio parahaemolyticus O3:K6 clone causing most associated diarrhea cases in the Pacific Northwest coast of Mexico[J]. Front Microbiol, 2015, 6: 221. |
| [15] |
Han DS, Yu F, Tang H, et al. Spreading of pandemic Vibrio parahaemolyticus O3:K6 and its serovariants:a re-analysis of strains isolated from multiple studies[J]. Front Cell Infect Microbiol, 2017, 7: 188. DOI:10.3389/fcimb.2017.00188 |
| [16] |
Xie TF, Xu XK, Wu QP, et al. Prevalence, molecular characterization, and antibiotic susceptibility of Vibrio parahaemolyticus from ready-to-eat foods in China[J]. Front Microbiol, 2016, 7: 549. DOI:10.3389/fmicb.2016.00549 |
| [17] |
Kang CH, Shin Y, Kim W, et al. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from oysters in Korea[J]. Environ Sci Pollut Res, 2016, 23(1): 918-926. DOI:10.1007/s11356-015-5650-9 |
| [18] |
Pazhani GP, Bhowmik SK, Ghosh S, et al. Trends in the epidemiology of pandemic and non-pandemic strains of Vibrio parahaemolyticus isolated from diarrheal patients in Kolkata, India[J]. PLoS Negl Trop Dis, 2014, 8(5): e2815. DOI:10.1371/journal.pntd.0002815 |
| [19] |
Letchumanan V, Pusparajah P, Tan LT, et al. Occurrence and antibiotic resistance of Vibrio parahaemolyticus from shellfish in Selangor, Malaysia[J]. Front Microbiol, 2015, 6: 1417. DOI:10.3389/fmicb.2015.01417 |
| [20] |
Letchumanan V, Yin WF, Lee LH, et al. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia[J]. Front Microbiol, 2015, 6: 33. DOI:10.3389/fmicb.2015.00033 |
| [21] |
Obaidat MM, Salman AEB, Roess AA. Virulence and antibiotic resistance of Vibrio parahaemolyticus isolates from seafood from three developing countries and of worldwide environmental, seafood, and clinical isolates from 2000 to 2017[J]. J Food Prot, 2017, 80(12): 2060-2067. DOI:10.4315/0362-028X.JFP-17-156 |
| [22] |
Xu XK, Cheng JH, Wu QP, et al. Prevalence, characterization, and antibiotic susceptibility of Vibrio parahaemolyticus isolated from retail aquatic products in North China[J]. BMC Microbiol, 2016, 16: 32. DOI:10.1186/s12866-016-0650-6 |
| [23] |
Ottaviani D, Leoni F, Talevi G, et al. Extensive investigation of antimicrobial resistance in Vibrio parahaemolyticus from shellfish and clinical sources, Italy[J]. Int J Antimicrob Agents, 2013, 42(2): 191-193. DOI:10.1016/j.ijantimicag.2013.05.003 |
| [24] |
李薇薇, 梅玲玲, 唐震, 等. 2007-2009年中国副溶血弧菌临床分离株分子特征分析[J]. 中华预防医学杂志, 2014, 48(1): 44-52. Li WW, Mei LL, Tang Z, et al. Analysis of molecular features of clinical Vibrio parahaemolyticus strains in China[J]. Chin J Prev Med, 2014, 48(1): 44-52. DOI:10.3760/cma.j.issn.0253-9624.2014.01.010 |
| [25] |
Chen Y, Chen X, Yu F, et al. Serology, virulence, antimicrobial susceptibility and molecular characteristics of clinical Vibrio parahaemolyticus strains circulating in southeastern China from 2009 to 2013[J]. Clin Microbiol Infect, 2016, 22(3): 258.e259-258.e216. DOI:10.1016/j.cmi.2015.11.003 |
 2018, Vol. 33
2018, Vol. 33


